Abstract
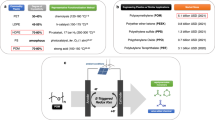
Petroleum-derived polyolefins exhibit diverse properties and are the most important and largest volume class of plastics. However, polyolefins are difficult to efficiently recycle or break down and are now a persistent global contaminant. Broadly replacing polyolefins with bio-derived and degradable polyethylene-like materials is an important yet challenging endeavour towards sustainable plastics. Here we report a solution for circular bio-based polyethylene-like materials synthesized by acceptorless dehydrogenative polymerization from linear and branched diols and their catalytic closed-loop recycling. The polymerization and depolymerization processes utilize earth-abundant manganese complexes as catalysts. These materials exhibit a wide range of mechanical properties, encompassing thermoplastics to plastomers to elastomers. The branched diols, produced through a thiol–ene click reaction, can be polymerized to plastics with significantly enhanced tensile properties, toughness and adhesive properties. These materials could be depolymerized back to monomers through hydrogenation and were separatable with a monomer recovery of up to 99%, unaffected by the presence of dyes and additives. Overall, this system establishes a route to more sustainable plastics.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Full experimental details and the summary of the data supporting the findings of this study are available within the article and its Supplementary Information. Owing to the large number of raw data files, they are available from the corresponding author on reasonable request. Source data are provided with this paper.
References
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, e1700782 (2017).
Galli, P. & Vecellio, G. Polyolefins: the most promising large-volume materials for the 21st century. J. Polym. Sci. 42, 396–415 (2004).
Westlie, A. H. et al. Polyolefin innovations toward circularity and sustainable alternatives. Macromol. Rapid Commun. 43, 2200492 (2022).
Qiao, J. et al. Recent advances in polyolefin technology. Polym. Chem. 2, 1611–1623 (2011).
Yeung, C. W. S., Teo, J. Y. Q., Loh, X. J. & Lim, J. Y. C. Polyolefins and polystyrene as chemical resources for a sustainable future: challenges, advances, and prospects. ACS Mater. Lett. 3, 1660–1676 (2021).
Chamas, A. et al. Degradation rates of plastics in the environment. ACS Sustain. Chem. Eng. 8, 3494–3511 (2020).
MacLeod, M., Arp, H. P. H., Tekman, M. B. & Jahnke, A. The global threat from plastic pollution. Science 373, 61–65 (2021).
Pabortsava, K. & Lampitt, R. S. High concentrations of plastic hidden beneath the surface of the atlantic ocean. Nat. Commun. 11, 4073 (2020).
Lebreton, L. & Andrady, A. Future scenarios of global plastic waste generation and disposal. Palgrave Commun. 5, 6 (2019).
Stubbins, A., Law, K. L., Muñoz, S. E., Bianchi, T. S. & Zhu, L. Plastics in the Earth system. Science 373, 51–55 (2021).
Jehanno, C. et al. Critical advances and future opportunities in upcycling commodity polymers. Nature 603, 803–814 (2022).
Zhao, X. et al. Plastic waste upcycling toward a circular economy. Chem. Eng. J. 428, 131928 (2022).
Chen, X., Wang, Y. & Zhang, L. Recent progress in the chemical upcycling of plastic wastes. ChemSusChem 14, 4137–4151 (2021).
Schwab, S. T., Baur, M., Nelson, T. F. & Mecking, S. Synthesis and deconstruction of polyethylene-type materials. Chem. Rev. 124, 2327–2351 (2024).
Li, X. et al. Sustainable high-density polyethylene via chemical recycling: from modification to polymerization methods. Polymer 295, 126698 (2024).
Lopez, G., Artetxe, M., Amutio, M., Bilbao, J. & Olazar, M. Thermochemical routes for the valorization of waste polyolefinic plastics to produce fuels and chemicals. A review. Renew. Sustain. Energ. Rev. 73, 346–368 (2017).
Galadima, A., Masudi, A. & Muraza, O. Towards low-temperature catalysts for sustainable fuel from plastic: a review. J. Environ. Chem. Eng. 9, 106655 (2021).
Arroyave, A. et al. Catalytic chemical recycling of post-consumer polyethylene. J. Am. Chem. Soc. 144, 23280–23285 (2022).
Kocen, A. L., Cui, S., Lin, T.-W., LaPointe, A. M. & Coates, G. W. Chemically recyclable ester-linked polypropylene. J. Am. Chem. Soc. 144, 12613–12618 (2022).
Jang, Y., Nguyen, S. & Hillmyer, M. A. Chemically recyclable linear and branched polyethylenes synthesized from stoichiometrically self-balanced telechelic polyethylenes. J. Am. Chem. Soc. 146, 4771–4782 (2024).
Han, X.-W. et al. Circular olefin copolymers made de novo from ethylene and α-olefins. Nat. Commun. 15, 1462 (2024).
Si, G., Li, C., Chen, M. & Chen, C. Polymer multi-block and multi-block+ strategies for the upcycling of mixed polyolefins and other plastics. Angew. Chem. Int. Ed. 62, e202311733 (2023).
Johnson, A. M. & Johnson, J. A. Thermally robust yet deconstructable and chemically recyclable high-density polyethylene (HDPE)-like materials based on Si–O bonds. Angew. Chem. Int. Ed. 135, e202315085 (2023).
Si, G. & Chen, C. Cyclic–acyclic monomers metathesis polymerization for the synthesis of degradable thermosets, thermoplastics and elastomers. Nat. Synth. 1, 956–966 (2022).
Häußler, M., Eck, M., Rothauer, D. & Mecking, S. Closed-loop recycling of polyethylene-like materials. Nature 590, 423–427 (2021).
Nelson, T. F., Rothauer, D., Sander, M. & Mecking, S. Degradable and recyclable polyesters from multiple chain length bio- and waste-sourceable monomers. Angew. Chem. Int. Ed. 62, e202310729 (2023).
Stille, J. K. Step-growth polymerization. J. Chem. Educ. 58, 862–866 (1981).
Zhao, Y. et al. Chemically recyclable polyolefin-like multiblock polymers. Science 382, 310–314 (2023).
Beuhler, A. A. ‘Game-changing’ C18 diacid for the plastics industry? Plast. Eng. 70, 32–35 (2014).
Chikkali, S. & Mecking, S. Refining of plant oils to chemicals by olefin metathesis. Angew. Chem. Int. Ed. 51, 5802–5808 (2012).
Gonzalez-de-Castro, A. et al. Long-chain α–ω diols from renewable fatty acids via tandem olefin metathesis–ester hydrogenation. Green Chem. 19, 1678–1684 (2017).
Hoyle, C. E. & Bowman, C. N. Thiol–ene click chemistry. Angew. Chem. Int. Ed. 49, 1540–1573 (2010).
Pepels, M. P. F., Koeken, R. A. C., der Linden, S. J. J., Heise, A. & Duchateau, R. Mimicking (linear) low-density polyethylenes using modified polymacrolactones. Macromolecules 48, 4779–479 (2015).
Nguyen, D. H. et al. Manganese pincer complexes for the base-free, acceptorless dehydrogenative coupling of alcohols to esters: development, scope, and understanding. ACS Catal. 7, 2022–2032 (2017).
Das, U. K., Ben-David, Y., Leitus, G., Diskin-Posner, Y. & Milstein, D. Dehydrogenative cross-coupling of primary alcohols to form cross-esters catalyzed by a manganese pincer complex. ACS Catal. 9, 479–484 (2019).
Chakraborty, S. et al. Well-defined iron catalysts for the acceptorless reversible dehydrogenation-hydrogenation of alcohols and ketones. ACS Catal. 4, 3994–4003 (2014).
Gandeepan, P. et al. 3d transition metals for C–H activation. Chem. Rev. 119, 2192–2452 (2019).
Ysart, G. et al. Dietary exposure estimates of 30 elements from the UK Total Diet Study. Food Addit. Contam. 16, 391–403 (1999).
Tran, Q. H., Neidhart, E. K., Sheiko, S. S., Bras, W. & Leibfarth, F. A. Polyolefin ionomer synthesis enabled by C–H thioheteroarylation. Macromolecules 56, 8920–8927 (2023).
Tsvankin, D. Y., Zubov, A. & Kitaigorodskii, A. I. Calculation of long periods in polymers and determination of the sizes of crystallites and amorphous regions. J. Polym. Sci. 16, 4081–4091 (1967).
Herrera, C., Ysinga, K. J. & Jenkins, C. L. Polysulfides synthesized from renewable garlic components and repurposed sulfur form environmentally friendly adhesives. ACS Appl. Mater. Interfaces 11, 35312–35318 (2019).
Ellison, M. M. & Taylor, L. T. Sulfur versus non-sulfur containing polyimide adhesives for bonding steel. J. Adhes. 60, 51–69 (1997).
Ragaert, K., Delva, L. & van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 69, 24–58 (2017).
Barnard, E., Arias, J. J. R. & Thielemans, W. Chemolytic depolymerisation of PET: a review. Green Chem. 23, 3765–3789 (2021).
Rosato, D. V. & Rosato, M. G. Injection Molding Handbook 3rd edn (Springer, 2000).
Acknowledgements
We thank Z. Zhang for insightful discussions throughout the lap-shear adhesion testing. This work was supported by the National Institutes of Health under award no. R35GM144356 and RePLACE (Redesigning Polymers to Leverage a Circular Economy) funded by the Office of Science of the US Department of Energy through award no. DE-SC0022290. Funding for N.A.R. and J.M. was provided in part by the US Department of Energy, Office of Energy Efficiency and Renewable Energy, Advanced Manufacturing Office (AMO) and Bioenergy Technologies Office (BETO). This work was performed as part of the BOTTLE Consortium and was supported by AMO and BETO under contract no. DE-AC36-08GO28308 with the National Renewable Energy Laboratory, operated by Alliance for Sustainable Energy, LLC. We thank the Analytical Resources Core (RRID: SCR_021759) and the SAMD Core (RRID: SCR_022002) at Colorado State University for instrument access and training. G.M.M. acknowledges support from The Camille & Henry Dreyfus Foundation through a Camille Dreyfus Teacher-Scholar Award and the Dr. Robert Williams Professorship in Organic Chemistry at Colorado State University.
Author information
Authors and Affiliations
Contributions
X.L., Z.H. and G.M.M. conceived the idea. X.L. and Z.H. designed and conducted the experiments and analysed results. X.L., Z.H., E.M.R., J.M. and N.A.R. performed characterization and analysed results. X.L. and K.L.H synthesized the required complexes. X.L. wrote the initial paper and supplementary materials. All authors read and edited the paper. G.M.M. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Georgina Gregory, Benjamin McDonald, Christophe Thomas and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–38, Discussion and Tables 1–17.
Source data
Source Data Fig. 2.
Statistical source data.
Source Data Fig. 3.
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, X., Hu, Z., Rettner, E.M. et al. Catalytic closed-loop recycling of polyethylene-like materials produced by acceptorless dehydrogenative polymerization of bio-derived diols. Nat. Chem. 17, 500–506 (2025). https://doi.org/10.1038/s41557-025-01753-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01753-8
This article is cited by
-
Synthesis of discrete oligoethylenes towards chemically recyclable polyolefins
Nature Synthesis (2026)
-
Recyclable plastics from a manganese catalyst
Nature Chemistry (2025)