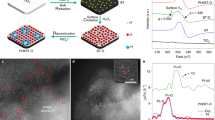
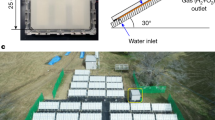
Abstract
Propane dehydrogenation is an energy-intensive industrial reaction that requires high temperatures (550–750 °C) to overcome thermodynamic barriers. Here we overcome these limits and demonstrate that near-ambient propane dehydrogenation can be achieved through photo-thermo-catalysis in a water-vapour environment. We reduce the reaction temperature to 50–80 °C using a single-atom catalyst of copper supported on TiO2 and a continuous-flow fixed-bed reactor. The mechanism differs from conventional propane dehydrogenation in that hydrogen is produced from the photocatalytic splitting of water vapour, surface-bound hydroxyl radicals extract propane hydrogen atoms to form propylene without over-oxidation, and water serves as a catalyst. This route also works for the dehydrogenation of other small alkanes. Moreover, we demonstrate sunlight-driven water-catalysed propane dehydrogenation operating at reaction temperatures as low as 10 °C. We anticipate that this work will be a starting point for integrating solar energy usage into a wide range of high-temperature industrial reactions.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data supporting the findings of this study are available within the Article and its Supplementary Information. Source data are provided with this paper.
References
Monai, M., Gambino, M., Wannakao, S. & Weckhuysen, B. M. Propane to olefins tandem catalysis: a selective route towards light olefins production. Chem. Soc. Rev. 50, 11503–11529 (2021).
Gao, X.-Q., Lu, W.-D., Hu, S.-Z., Li, W.-C. & Lu, A.-H. Rod-shaped porous alumina-supported Cr2O3 catalyst with low acidity for propane dehydrogenation. Chin. J. Catal. 40, 184–191 (2019).
Motagamwala, A. H., Almallahi, R., Wortman, J., Igenegbai, V. O. & Linic, S. Stable and selective catalysts for propane dehydrogenation operating at thermodynamic limit. Science 373, 217–222 (2021).
Liu, L. et al. Regioselective generation and reactivity control of subnanometric platinum clusters in zeolites for high-temperature catalysis. Nat. Mater. 18, 866–873 (2019).
Zhao, D. et al. In situ formation of ZnOx species for efficient propane dehydrogenation. Nature 599, 234–238 (2021).
Ryoo, R. et al. Rare-earth-platinum alloy nanoparticles in mesoporous zeolite for catalysis. Nature 585, 221–224 (2020).
Yang, W., Lin, L., Fan, Y. & Zang, J. Surface-structure and catalytic performance of supported PtSn catalysts. Catal. Lett. 12, 267–276 (1992).
Gomez, E. et al. Combining CO2 reduction with propane oxidative dehydrogenation over bimetallic catalysts. Nat. Commun. 9, 1398 (2018).
Yan, H. et al. Tandem In2O3-Pt/Al2O3 catalyst for coupling of propane dehydrogenation to selective H2 combustion. Science 371, 1257–1260 (2021).
Zhang, J., Ma, R., Ham, H., Shimizu, K.-i & Furukawa, S. Electroassisted propane dehydrogenation at low temperatures: far beyond the equilibrium limitation. JACS Au 1, 1688–1693 (2021).
Chen, S. et al. Propane dehydrogenation: catalyst development, new chemistry and emerging technologies. Chem. Soc. Rev. 50, 3315–3354 (2021).
Grant, J. T. et al. Selective oxidative dehydrogenation of propane to propene using boron nitride catalysts. Science 354, 1570–1573 (2016).
Cavani, F., Ballarini, N. & Cericola, A. Oxidative dehydrogenation of ethane and propane: how far from commercial implementation? Catal. Today 127, 113–131 (2007).
Vajda, S. et al. Subnanometre platinum clusters as highly active and selective catalysts for the oxidative dehydrogenation of propane. Nat. Mater. 8, 213–216 (2009).
Wang, Y. et al. Roles of catalyst structure and gas surface reaction in the generation of hydroxyl radicals for photocatalytic oxidation. ACS Catal. 12, 2770–2780 (2022).
Han, C. et al. Selective cleavage of chemical bonds in targeted intermediates for highly selective photooxidation of methane to methanol. J. Am. Chem. Soc. 145, 8609–8620 (2023).
Devaraji, P., Sathu, N. K. & Gopinath, C. S. Ambient oxidation of benzene to phenol by photocatalysis on Au/Ti0.98V0.02O2: role of holes. ACS Catal. 4, 2844–2853 (2014).
Park, H. & Choi, W. Photocatalytic conversion of benzene to phenol using modified TiO2 and polyoxometalates. Catal. Today 101, 291–297 (2005).
Du, P., Moulijn, J. A. & Mul, G. Selective photo(catalytic)-oxidation of cyclohexane: effect of wavelength and TiO2 structure on product yields. J. Catal. 238, 342–352 (2006).
Estahbanati, M. R. K. et al. Selective photocatalytic oxidation of cyclohexanol to cyclohexanone: a spectroscopic and kinetic study. Chem. Eng. J. 382, 122732 (2020).
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–801 (2016).
Cheng, C., Fang, W.-H., Long, R. & Prezhdo, O. V. Water splitting with a single-atom Cu/TiO2 photocatalyst: atomistic origin of high efficiency and proposed enhancement by spin selection. JACS Au 1, 550–559 (2021).
Zhang, Y. et al. Single-atom Cu anchored catalysts for photocatalytic renewable H2 production with a quantum efficiency of 56. Nat. Commun. 13, 58 (2022).
Nishiyama, H. et al. Photocatalytic solar hydrogen production from water on a 100-m2 scale. Nature 598, 304–307 (2021).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Zhao, Y. et al. Solar-versus thermal-driven catalysis for energy conversion. Joule 3, 920–937 (2019).
Wang, Z., Song, H., Liu, H. & Ye, J. Coupling of solar energy and thermal energy for carbon dioxide reduction: status and prospects. Angew. Chem. Int. Ed. 59, 8016–8035 (2020).
Ohtani, B., Prieto-Mahaney, O. O., Li, D. & Abe, R. What is Degussa (Evonik) P25? Crystalline composition analysis, reconstruction from isolated pure particles and photocatalytic activity test. J. Photochem. Photobiol. A Chem. 216, 179–182 (2010).
Liu, L., Gao, F., Zhao, H. & Li, Y. Tailoring Cu valence and oxygen vacancy in Cu/TiO2 catalysts for enhanced CO2 photoreduction efficiency. Appl. Catal. B Environ. 134, 349–358 (2013).
Imai, S. et al. 63Cu NMR study of copper (I) carbonyl complexes with various hydrotris(pyrazolyl)borates: correlation between 63Cu chemical shifts and CO stretching vibrations. Inorg. Chem. 37, 3066–3070 (1998).
DeRita, L. et al. Catalyst architecture for stable single atom dispersion enables site-specific spectroscopic and reactivity measurements of CO adsorbed to Pt atoms, oxidized Pt clusters and metallic Pt clusters on TiO2. J. Am. Chem. Soc. 139, 14150–14165 (2017).
Neubert, S. et al. Highly efficient rutile TiO2 photocatalysts with single Cu(II) and Fe(III) surface catalytic sites. J. Mater. Chem. A 4, 3127–3138 (2016).
Lee, B.-H. et al. Reversible and cooperative photoactivation of single-atom Cu/TiO2 photocatalysts. Nat. Mater. 18, 620–626 (2019).
Hurum, D. C., Agrios, A. G., Gray, K. A., Rajh, T. & Thurnauer, M. C. Explaining the enhanced photocatalytic activity of Degussa P25 mixed-phase TiO2 using EPR. J. Phys. Chem. B 107, 4545–4549 (2003).
Li, G. et al. The important role of tetrahedral Ti4+ sites in the phase transformation and photocatalytic activity of TiO2 nanocomposites. J. Am. Chem. Soc. 130, 5402–5403 (2008).
Zhao, Y. et al. A hydrogen farm strategy for scalable solar hydrogen production with particulate photocatalysts. Angew. Chem. Int. Ed. 59, 9653–9658 (2020).
Hisatomi, T., Maeda, K., Takanabe, K., Kubota, J. & Domen, K. Aspects of the water splitting mechanism on (Ga1–xZnx)(N1–xOx) photocatalyst modified with Rh2−yCryO3 cocatalyst. J. Phys. Chem. C 113, 21458–21466 (2009).
Nakabayashi, S., Fujishima, A. & Honda, K. Experimental-evidence for the hydrogen evolution site in photocatalytic process on Pt/TiO2. Chem. Phys. Lett. 102, 464–465 (1983).
Baba, R., Nakabayashi, S., Fujishima, A. & Honda, K. Investigation of the mechanism of hydrogen evolution during photocatalytic water decomposition on metal-loaded semiconductor powders. J. Phys. Chem. 89, 1902–1905 (1985).
Jiang, Y. et al. Elevating photooxidation of methane to formaldehyde via TiO2 crystal phase engineering. J. Am. Chem. Soc. 144, 15977–15987 (2022).
Lin, X. et al. Solvent-mediated precipitating synthesis and optical properties of polyhydrido Cu13 nanoclusters with four vertex-sharing tetrahedrons. Chem. Sci. 14, 994–1002 (2023).
Kang, L. et al. Photo-thermo catalytic oxidation over TiO2-WO3 supported platinum catalyst. Angew. Chem. Int. Ed. 59, 12909–12916 (2020).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics for liquid-metals. Phys. Rev. B 47, 558–561 (1993).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P. et al. Atoms, molecules, solids, and surfaces: applications of the generalized gradient approximation for exchange and correlation. Phys. Rev. B 46, 6671–6687 (1992).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Henkelman, G. & Jonsson, H. A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives. J. Chem. Phys. 111, 7010–7022 (1999).
Henkelman, G., Uberuaga, B. P. & Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Henkelman, G. & Jonsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Fu, Y. et al. dz2 band links frontier orbitals and charge carrier dynamics of single-atom cocatalyst-aided photocatalytic H2 production. J. Am. Chem. Soc. 145, 28166–28175 (2023).
Acknowledgements
This work was supported financially by the National Key R&D Program of China (2023YFA1507800 to X.Y.L. and L.K.), the NSFC Center for Single-Atom Catalysis (22388102 to A.W. and T.Z.), the National Natural Science Foundation of China (22102180 to L.K. and 22472164 to B.Y.), the DNL Cooperation Fund, CAS (DNL202002 to X.Y.L.), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB0540000 to X.Y.L., J.X. and G.Q.), the Key Research Program of Frontier Sciences, CAS (ZDBS-LY-7012 to B.Z.), CAS Project for Young Scientists in Basic Research (YSBR-022 to B.Y.), Youth Innovation Promotion Association, CAS (Y201828 to B.Z.), LiaoNing Revitalization Talents Program (XLYC2007070 to X.Y.L.), Fundamental Research Funds for the Central Universities (20720220009 to X.Y.L.), the Natural Science Foundation of Shanghai Municipality (22JC1404200 to Y.G.), Shanghai Municipal Science and Technology Major Project and the Foundation of Key Laboratory of Low-Carbon Conversion Science & Engineering, Shanghai Advanced Research Institute, Chinese Academy of Sciences (KLLCCSE-202201Z to Y.G.). We acknowledge support from the National Supercomputing Center in Guangzhou (NSCC-GZ), Shanghai and Tianjin. C. Liu is thanked for providing the Cu cluster sample.
Author information
Authors and Affiliations
Contributions
L.K. performed the catalyst preparation, characterization and catalytic tests. Y.G., B.Z., X.D. and L.Y. carried out the theoretical calculations. B.Y., Q.G. and Y.S. performed the AC-HAADF-STEM measurements. L.L. and Y.X. performed the DRIFTS measurements. G.Q. and J.X. performed the NMR experiments and analysis. G.L. and Y.W. helped to build up the continuous-flow, fixed-bed, stainless-steel reactor. L.K., X.Y.L., B.Z., Y.G., B.Y. and R.L. analysed the data, and wrote and revised the paper. X.Y.L., A.W. and T.Z. designed the study.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Lyudmila Matteo Monai, V. Moskaleva and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–40, Tables 1 and 2 and reaction equations.
Supplementary Data 1
TCD and FID signals of gas chromatographs of exhaust gases.
Supplementary Data 2
In situ CO DRIFTS on Cu1/TiO2-fresh in the dark.
Supplementary Data 3
In situ CO DRIFTS on Cu1/TiO2-activated in the dark.
Supplementary Data 4
In situ CO DRIFTS on Cu1/TiO2-activated under light illumination.
Supplementary Data 5
Photoluminescence spectra.
Supplementary Data 6
Online MS signals of HDO and D2O when using C3D8 and H2O as the reactants.
Supplementary Data 7
X-ray photoelectron spectra.
Supplementary Data 8
In situ DRIFTS spectra of the Cu1/TiO2-activated.
Supplementary Data 9
In situ DRIFTS spectra of the Cu1/SrTiO3:Al.
Supplementary Data 10
In situ CO DRIFTS on Cu1/SrTiO3:Al in the dark.
Supplementary Data 11
In situ CO DRIFTS on Cu1/SrTiO3:Al under light illumination.
Supplementary Data 12
In situ DRIFTS spectra of the Cu1/TiO2-activated when introducing propylene.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kang, L., Zhu, B., Gu, Q. et al. Light-driven propane dehydrogenation by a single-atom catalyst under near-ambient conditions. Nat. Chem. 17, 890–896 (2025). https://doi.org/10.1038/s41557-025-01766-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01766-3
This article is cited by
-
Harnessing light for propane dehydrogenation: a single-atom catalyst milestone achieved under mild conditions
Science China Chemistry (2025)