Abstract
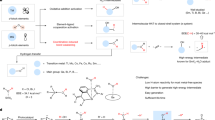
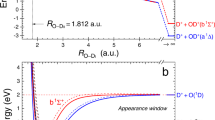
The mutual neutralization of hydronium and hydroxide ions is a fundamental chemical reaction. Yet, there is very limited direct experimental evidence about its intrinsically non-adiabatic mechanism. Chemistry textbooks describe the products of mutual neutralization in bulk water as two water molecules; however, this reaction has been suggested as a possible mechanism for the recently reported spontaneous formation of OH radicals at the surface of water microdroplets. Here, following three-dimensional-imaging of the coincident neutral products of reactions of isolated D3O+ and OD−, we can reveal the non-adiabatic pathways for OD radical formation. Two competing pathways lead to distinct D2O + OD + D and 2OD + D2 product channels, while the proton-transfer mechanism is substantially suppressed due to a kinetic isotope effect. Analysis of the three-body momentum correlations revealed that the D2O + OD + D channel is formed by electron transfer at a short distance of ~4 Å with the formation of the intermediate unstable neutral D3O ground state, while 2OD + D2 products are obtained following electron transfer at a distance of ~10 Å via an excited state of the neutral D3O.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Source data are provided with this paper.
References
Schuurman, M. S. & Stolow, A. Dynamics at conical intersections. Annu. Rev. Phys. Chem. 69, 427–450 (2018).
Mai, S., Marquetand, P. & González, L. A general method to describe intersystem crossing dynamics in trajectory surface hopping. Int. J. Quantum Chem. 115, 1215–1231 (2015).
Penfold, T. J., Gindensperger, E., Daniel, C. & Marian, C. M. Spin-vibronic mechanism for intersystem crossing. Chem. Rev. 118, 6975–7025 (2018).
Schnorr, K. et al. Direct tracking of ultrafast proton transfer in water dimers. Sci. Adv. 9, eadg7864 (2023).
Luzon, I., Livshits, E., Gope, K., Baer, R. & Strasser, D. Making sense of Coulomb explosion imaging. J. Phys. Chem. Lett. 10, 1361–1367 (2019).
Livshits, E., Luzon, I., Gope, K., Baer, R. & Strasser, D. Time-resolving the ultrafast H2 roaming chemistry and H3+ formation using extreme-ultraviolet pulses. Commun. Chem. 3, 49 (2020).
Gope, K., Livshits, E., Bittner, D. M., Baer, R. & Strasser, D. An “inverse” harpoon mechanism. Sci. Adv. 8, 2–9 (2022).
Yu, H. G. Spherical electron cloud hopping molecular dynamics simulation on dissociative recombination of protonated water. J. Phys. Chem. A 113, 6555–6561 (2009).
Kayanuma, M., Taketsugu, T. & Ishii, K. Ab initio surface hopping simulation on dissociative recombination of H3O+. Chem. Phys. Lett. 418, 511–518 (2006).
Hassanali, A., Prakash, M. K., Eshet, H. & Parrinello, M. On the recombination of hydronium and hydroxide ions in water. Proc. Natl Acad. Sci. USA 108, 20410–20415 (2011).
Geissler, P. L., Dellago, C., Chandler, D., Hutter, J. & Parrinello, M. Autoionization in liquid water. Science 291, 2121–2124 (2001).
Eklund, G. et al. Cryogenic merged-ion-beam experiments in DESIREE: final-state-resolved mutual neutralization of Li+ and D−. Phys. Rev. A 102, 12823 (2020).
Bogot, A. et al. The mutual neutralization of hydronium and hydroxide. Science 383, 285–289 (2024).
Poline, M. et al. Final-state-resolved mutual neutralization in I+–I− collisions. Phys. Rev. A 106, 012812 (2022).
Poline, M. et al. Mutual neutralization of NO+ with O−. Phys. Rev. Lett. 132, 023001 (2024).
Dochain, A., Andrianarijaona, V. M. & Urbain, X. Isotope effect for the mutual neutralization reaction at low collision energies: He+ + H−. Phys. Rev. A 108, 042809 (2023).
Schmidt-May, A. F. et al. Observation of an isotope effect in state-selective mutual neutralization of lithium with hydrogen. Phys. Rev. A 108, 042810 (2023).
Schmidt, H. T. et al. Rotationally cold OH− ions in the cryogenic electrostatic ion-beam storage ring DESIREE. Phys. Rev. Lett. 119, 079901 (2017).
Bogot, A., Lioubashevski, O., Heber, O., Zajfman, D. & Strasser, D. Simultaneous electrostatic trapping of merged cation & anion beams. Phys. Chem. Chem. Phys. 25, 25701–25710 (2023).
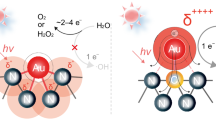
Hao, H., Leven, I. & Head-Gordon, T. Can electric fields drive chemistry for an aqueous microdroplet? Nat. Commun. 13, 280 (2022).
Lee, J. K. et al. Spontaneous generation of hydrogen peroxide from aqueous microdroplets. Proc. Natl Acad. Sci. USA 116, 19294–19298 (2019).
Mehrgardi, M. A., Mofidfar, M. & Zare, R. N. Sprayed water microdroplets are able to generate hydrogen peroxide spontaneously. J. Am. Chem. Soc. 144, 7606–7609 (2022).
Skurski, P. & Simons, J. Two potential paths for OH radical formation on surfaces of pure water microdroplets. J. Chem. Phys. 160, 034708 (2024).
Li, K. et al. Spontaneous dark formation of OH radicals at the interface of aqueous atmospheric droplets. Proc. Natl Acad. Sci. USA 120, 10868–10874 (2023).
Chen, X. et al. Sprayed oil–water microdroplets as a hydrogen source. J. Am. Chem. Soc. 146, 10868–10874 (2024).
Smith, J. R., Kim, J. B. & Lineberger, W. C. High-resolution threshold photodetachment spectroscopy of OH−. Phys. Rev. A 55, 2036–2043 (1997).
Luo, M. & Jungen, M. The H3O Rydberg radical. Chem. Phys. 241, 297–303 (1999).
Ketvirtis, A. E. & Simons, J. Dissociative recombination of H3O+. J. Phys. Chem. 103, 6552–6563 (1999).
Hvelplund, P., Nielsen, S. B., Panja, S., Pedersen, J. O. P. & Uggerud, E. On the existence of the hypervalent H3O, H2DO, HD2O, and D3O radicals. Int. J. Mass spectrom. 281, 52–54 (2009).
Andersen, L. H. et al. Production of water molecules from dissociative recombination of H3O+ with electrons. Phys. Rev. Lett. 77, 4891–4894 (1996).
Mann, J. E., Xie, Z., Savee, J. D., Bowman, J. M. & Continetti, R. E. Production of vibrationally excited H2O from charge exchange of H3O+ with cesium. J. Chem. Phys. 130, 041102 (2009).
Mann, J. E., Xie, Z., Savee, J. D., Bowman, J. M. & Continetti, R. E. Vibrational excitation and product branching ratios in dissociation of the isotopologs of H3O: experiment and theory. J. Phys. Chem. A 117, 7256–7266 (2013).
Buhr, H. et al. Hot water molecules from dissociative recombination of D3O+ with cold electrons. Phys. Rev. Lett. 105, 103202 (2010).
Stillinger, F. H. & Weber, T. A. Polarization model study of isotope effects in the gas phase hydronium–hydroxide neutralization reaction. J. Chem. Phys. 76, 4028–4036 (1982).
Kiefer, P. M. & Hynes, J. T. Kinetic isotope effects for adiabatic proton transfer reactions in a polar environment. J. Phys. Chem. A 107, 9022–9039 (2003).
Tiwari, A. K. & Sathyamurthy, N. Preferential scattering of one isotopomer over another in (He, HD+) collisions. Chem. Phys. Lett. 414, 509–513 (2005).
Grussie, F. et al. Merged-beams study of the reaction of cold HD+ with C atoms reveals a pronounced intramolecular kinetic isotope effect. Phys. Rev. Lett. 132, 243001 (2024).
Ringnér, M. What is principal component analysis? Nat. Biotechnol. 26, 303–304 (2008).
Dalitz, R. H. On the analysis of τ-meson data and the nature of the τ-meson. Lond. Edinb. Dubl. Phil. Mag. J. Sci. 44, 1068–1080 (1953).
Strasser, D. et al. Breakup dynamics and the isotope effect in H3+ and D3+ dissociative recombination. Phys. Rev. A 66, 032719 (2002).
Laperle, C. M., Mann, J. E., Clements, T. G. & Continetti, R. E. Three-body dissociation dynamics of the low-lying Rydberg states of H3 and D3. Phys. Rev. Lett. 93, 153202 (2004).
Strasser, D. et al. Breakup dynamics and isotope effects in D2H+ and H2D+ dissociative recombination. Phys. Rev. A 69, 064702 (2004).
Gope, K., Luzon, I. & Strasser, D. N–NO & NN–O bond cleavage dynamics in two- and three-body Coulomb explosion of the N2O2+ dication. Phys. Chem. Chem. Phys. 21, 13730–13737 (2019).
Bittner, D. M., Gope, K., Livshits, E., Baer, R. & Strasser, D. Sequential and concerted C–C and C–O bond dissociation in the Coulomb explosion of 2-propanol. J. Chem. Phys. 157, 074309 (2022).
Gope, K., Bittner, D. M. & Strasser, D. Sequential mechanism in H3+ formation dynamics on the ethanol dication. Phys. Chem. Chem. Phys. 25, 6979–6986 (2023).
Gellene, G. I. & Porter, R. F. Experimental evidence for metastable states of D3O and its monohydrate by neutralized ion beam spectroscopy. J. Chem. Phys. 81, 5570–5576 (1984).
Blais, N. C. Monte Carlo trajectories: the dynamics of harpooning in alkali–halogen reactions. J. Chem. Phys. 49, 9–14 (1968).
Zener, C. Non-adiabatic crossing of energy levels. Proc. R. Soc. London. A 137, 696–702 (1932).
Ben Abu, N. et al. Sweet taste of heavy water. Commun. Biol. 4, 440 (2021).
Stockett, M. H. et al. Efficient stabilization of cyanonaphthalene by fast radiative cooling and implications for the resilience of small PAHs in interstellar clouds. Nat. Commun. 14, 395 (2023).
Thomas, R. D. et al. The double electrostatic ion ring experiment: a unique cryogenic electrostatic storage ring for merged ion-beams studies. Rev. Sci. Instrum. 82, 065112 (2011).
Schmidt, H. T. et al. First storage of ion beams in the double electrostatic ion-ring experiment: DESIREE. Rev. Sci. Instrum. 84, 055115 (2013).
Source of negative ions by cesium sputtering (SNICS). National Electrostatic Corporation https://www.pelletron.com/products/snics/
Fisher-Levine, M. & Nomerotski, A. TimepixCam: a fast optical imager with time-stamping. J. Instrum. 11, C03016 (2016).
Nomerotski, A. Imaging and time stamping of photons with nanosecond resolution in Timepix based optical cameras. Nucl. Instrum. Methods Phys. Res. A 937, 26–30 (2019).
Acknowledgements
This work was performed at the Swedish National Infrastructure, DESIREE (Swedish Research Council contract nos. 2017-00621, 2021-00155 and 2023-00170). H.Z. and H.T.S. thank the Swedish Research Council for individual project grants (contract nos. 2020-03437 and 2022-02822). R.D.T., H.T.S. and H.Z. acknowledge the Project Grant ‘Probing charge- and mass-transfer reactions on the atomic level’ (2018.0028) from the Knut and Alice Wallenberg Foundation. R.D.T. acknowledges support by the Air Force Office of Scientific Research (award no. FA8655-24-1-7004). This publication is based upon work from COST Action CA18212—Molecular Dynamics in the GAS phase (MD-GAS), supported by COST (European Cooperation in Science and Technology). D.S. and A.B. acknowledge support from ISF grant 674/21 and the Minerva Center for Making Bonds by Fragmentation.
Author information
Authors and Affiliations
Contributions
All authors contributed to the reported investigations and participated in the experimental measurements. D.S., H.T.S., H.Z. and R.D.T. acquired the funding and supervised the research. A.B. and D.S. wrote the original draft of the paper and A.B., M.P., A.D., H.Z., H.T.S., R.D.T. and D.S. reviewed and edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–7, Figs. 1–10 and Tables 1 and 2.
Supplementary Data 1
Source data for Supplementary Figs. 2–6 and 8–10.
Source data
Source Data Figs. 1, 3 and 4
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bogot, A., Poline, M., Ji, M. et al. Unravelling non-adiabatic pathways in the mutual neutralization of hydronium and hydroxide. Nat. Chem. 17, 541–546 (2025). https://doi.org/10.1038/s41557-025-01771-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01771-6