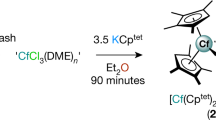
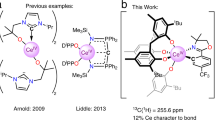
Abstract
Metal–ligand bonding interactions for f-element compounds are typically highly polarized with only minor covalent character. Whereas the 5d/6d orbitals are known to be chemically accessible for dative bonding, recent quantum chemical and spectroscopic analyses have indicated appreciable 4f/5f-orbital involvement in certain metal–ligand bonds. However, 4f-orbital covalency has not been compellingly linked to distinctive modes of chemical reactivity via rigorous comparative study and mechanistic investigation. Here a series of MIV–cyclopropenyl complexes (M = Ti, Zr, Ce, Hf, Th) are described, wherein the cerium congener exhibits a 4f-covalent Ce=Cα interaction, causing a ring-opening isomerization reaction through a single-crystal-to-single-crystal transformation. The results provide evidence for 4f-orbital covalency by demonstrating its expression in the reactivity of an f-element complex within an isostructural series of tetravalent d- and f-block metal complexes. They also provide new directions for the study of orbital covalency effects of molecular compounds in solid-state chemical transformations.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data that support the findings of this study are available within the Article and its Supplementary Information. Crystallographic data reported in this paper are tabulated in the Supplementary Information and archived at the Cambridge Crystallographic Data Centre with the following codes: Ce-1∙C6H5F (CCDC 2355960), Zr-1∙C6H5CH3 (CCDC 2355961), Zr-0 (CCDC 2355962), Ce-1∙C6H6 (CCDC 2355963), Th-1∙C6H5CH3 (CCDC 2355964), Ce-1∙C6H5CH3 (CCDC 2355965), Ce-1∙Mes (CCDC 2355966), Hf-1∙C5H12 (CCDC 2355967), Ce-2∙C6H5CH3 (CCDC 2355968), Ti-1 (CCDC 2355969), Ce-1∙C6H5CH3-49d5 (CCDC 2357992), Ce-1∙C6H5CH3-215d5 (CCDC 2357993), Ce-1∙C6H5CH3-568d5 (CCDC 2357994), Ce-1∙C6H5CH3-913d5 (CCDC 2357995), Ce-1∙C6H5CH3-1263d5 (CCDC 2357996), Ce-1∙C6H5CH3-1644d5 (CCDC 2357997), Ce-1∙C6H5CH3-2009d5 (CCDC 2357998), Ce-1∙C6H5CH3-2353d5 (CCDC 2357999), Ce-1∙C6H5CH3-2715d5 (CCDC 2358000), Ce-1∙C6H5CH3-3089d5 (CCDC 2358001), Ce-1∙C6H5CH3-3459d5 (CCDC 2358002), Ce-1∙C6H5CH3-3834d5 (CCDC 2358003), Ce-1∙C6H5CH3-4206d5 (CCDC 2358004), Ce-1∙C6H5CH3-4576d5 (CCDC 2358005), Ce-1∙C6H5CH3-4943d5 (CCDC 2358006), Ce-1∙C6H5CH3-5321d5 (CCDC 2358007), Ce-1∙C6H5CH3-5684d5 (CCDC 2358008), Ce-1∙C6H5CH3-6040d5 (CCDC 2358009), Ce-1∙C6H5CH3-6408d5 (CCDC 2358010), Ce-1∙C6H5CH3-6771d5 (CCDC 2358011), Ce-1∙C6H5CH3-7133d5 (CCDC 2358012), Ce-1∙C6H5CH3-7491d5 (CCDC 2358013), Ce-1∙C6H5CH3-7845d5 (CCDC 2358014), Ce-1∙C6H5CH3-8203d5 (CCDC 2358015), Ce-1∙C6H5CH3-8565d5 (CCDC 2358016), Ce-1∙C6H5CH3-8910d5 (CCDC 2358017), Ce-1∙C6H5CH3-9266d5 (CCDC 2358018), Ce-1∙C6H5CH3-9637d5 (CCDC 2358019), Ce-1∙C6H5CH3-10025d5 (CCDC 2358020), Ce-1∙C6H5CH3-10401d5 (CCDC 2358021), Ce-1∙C6H5CH3-10772d5 (CCDC 2358022), Ce-1∙C6H5CH3-11161d5 (CCDC 2358023), Ce-1∙C6H5CH3-11528d5 (CCDC 2358024), Ce-1∙C6H5CH3-11894d5 (CCDC 2358025), Ce-1∙C6H5CH3-12265d5 (CCDC 2358026), Ce-1∙C6H5CH3-12643d5 (CCDC 2358027), Ce-1∙C6H5CH3-13013d5 (CCDC 2358028), Ce-1∙C6H5CH3-13385d5 (CCDC 2358029), Ce-1∙C6H5CH3-13751d5 (CCDC 2358030), Ce-1∙C6H5CH3-14104d5 (CCDC 2358031), Ce-1∙C6H5CH3-14470d5 (CCDC 2358032), Ce-1∙C6H5CH3-14821d5 (CCDC 2358033), Ce-1∙C6H5CH3-15180d5 (CCDC 2358034), Ce-1∙C6H5CH3-15524d5 (CCDC 2358035), Ce-1∙C6H5CH3-15874d5 (CCDC 2358036), Ce-1∙C6H5CH3-16227d5 (CCDC 2358037), Ce-1∙C6H5CH3-16579d5 (CCDC 2358038), Ce-1∙C6H5CH3-16932d5 (CCDC 2358039), Ce-1∙C6H5CH3-17284d5 (CCDC 2358040), Ce-1∙C6H5CH3-17635d5 (CCDC 2358041), Ce-1∙C6H5CH3-17988d5 (CCDC 2358042), Ce-1∙C6H5CH3-18343d5 (CCDC 2358043), Ce-1∙C6H5CH3-18695d5 (CCDC 2358044), Ce-1∙C6H5CH3-18796d5 (CCDC 2358045), Ce-1∙C6H5CH3-19141d5 (CCDC 2358046), Ce-1∙C6H5CH3-19496d5 (CCDC 2358047), Ce-1∙C6H5CH3-19855d5 (CCDC 2358048) and Ce-1∙C6H5CH3-20207d5 (CCDC 2358049). Source data are provided with this paper.
References
Charalampides, G., Vatalis, K. I., Apostoplos, B. & Ploutarch-Nikolas, B. Rare earth elements: industrial applications and economic dependency of Europe. Procedia Econ. Finance 24, 126–135 (2015).
Nelson, J. J. M. & Schelter, E. J. Sustainable inorganic chemistry: metal separations for recycling. Inorg. Chem. 58, 979–990 (2019).
Bünzli, J.-C. G. & Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 34, 1048–1077 (2005).
Woodruff, D. N., Winpenny, R. E. P. & Layfield, R. A. Lanthanide single-molecule magnets. Chem. Rev. 113, 5110–5148 (2013).
Qiao, Y. & Schelter, E. J. Lanthanide photocatalysis. Acc. Chem. Res. 51, 2926–2936 (2018).
Panetti, G. B. et al. Isolation and characterization of a covalent CeIV-Aryl complex with an anomalous 13C chemical shift. Nat. Commun. 12, 1713 (2021).
Gregson, M. et al. A cerium(IV)–carbon multiple bond. Angew. Chem. Int. Ed. 52, 13016–13019 (2013).
Gregson, M. et al. The inverse-trans-influence in tetravalent lanthanide and actinide bis(carbene) complexes. Nat. Commun. 8, 14137 (2017).
Casely, I. J., Liddle, S. T., Blake, A. J., Wilson, C. & Arnold, P. L. Tetravalent cerium carbene complexes. Chem. Commun. 5037–5039 (2007).
Tateyama, H. et al. Tetravalent cerium alkyl and benzyl complexes. J. Am. Chem. Soc. 146, 10268–10273 (2024).
Löble, M. W. et al. Covalency in lanthanides. An X-ray absorption spectroscopy and density functional theory study of LnCl6x− (x = 3, 2). J. Am. Chem. Soc. 137, 2506–2523 (2015).
Denning, R. G., Harmer, J., Green, J. C. & Irwin, M. Covalency in the 4f shell of tris-cyclopentadienyl ytterbium (YbCp3)—a spectroscopic evaluation. J. Am. Chem. Soc. 133, 20644–20660 (2011).
Ordoñez, O., Yu, X., Wu, G., Autschbach, J. & Hayton, T. W. Assessing the 4f orbital participation in the Ln–C bonds of [Li(THF)4][Ln(C6Cl5)4] (Ln = La, Ce). Inorg. Chem. 61, 15138–15143 (2022).
Tricoire, M., Mahieu, N., Simler, T. & Nocton, G. Intermediate valence states in lanthanide compounds. Chem. Eur. J. 27, 6860–6879 (2021).
Sergentu, D.-C., Booth, C. H. & Autschbach, J. Probing multiconfigurational states by spectroscopy: the cerium XAS L3-edge puzzle. Chem. Eur. J. 27, 7239–7251 (2021).
Smiles, D. E. et al. The duality of electron localization and covalency in lanthanide and actinide metallocenes. Chem. Sci. 11, 2796–2809 (2020).
Minasian, S. G. et al. Quantitative evidence for lanthanide–oxygen orbital mixing in CeO2, PrO2, and TbO2. J. Am. Chem. Soc. 139, 18052–18064 (2017).
Cheisson, T. et al. Multiple bonding in lanthanides and actinides: direct comparison of covalency in thorium(IV)- and cerium(IV)-imido complexes. J. Am. Chem. Soc. 141, 9185–9190 (2019).
Wilson, H. H. et al. Synthesis and characterization of a bridging cerium(IV) nitride complex. J. Am. Chem. Soc. 145, 781–786 (2023).
Solola, L. A. et al. Cerium(IV) imido complexes: structural, computational, and reactivity studies. J. Am. Chem. Soc. 139, 2435–2442 (2017).
Rieser, T. E. et al. Open-shell early lanthanide terminal imides. J. Am. Chem. Soc. 144, 4102–4113 (2022).
Kent, G. T., Staun, S. L., Wu, G. & Hayton, T. W. Reactivity of [Ce(NR2)3] (R = SiMe3) with prospective carbon atom transfer reagents. Organometallics 39, 2375–2382 (2020).
Chan, H.-S., Li, H.-W. & Xie, Z. Synthesis and structural characterization of imido–lanthanide complexes with a metal–nitrogen multiple bond. Chem. Commun. 652–653 (2002).
Rieser, T. E., Schädle, D., Maichle-Mössmer, C. & Anwander, R. Terminal dysprosium and holmium organoimides. Chem. Sci. 15, 3562–3570 (2024).
Kent, G. T., Yu, X., Wu, G., Autschbach, J. & Hayton, T. W. Ring-opening of a thorium cyclopropenyl complex generates a transient thorium-bound carbene. Chem. Commun. 58, 6805–6808 (2022).
Kent, G. T., Yu, X., Wu, G., Autschbach, J. & Hayton, T. W. Synthesis and electronic structure analysis of the actinide allenylidenes, [{(NR2)3}An(CCCPh2)]− (An = U, Th; R = SiMe3). Chem. Sci. 12, 14383–14388 (2021).
Bogart, J. A., Lippincott, C. A., Carroll, P. J., Booth, C. H. & Schelter, E. J. Controlled redox chemistry at cerium within a tripodal nitroxide ligand framework. Chem. Eur. J. 21, 17850–17859 (2015).
Boreen, M. A., Bogart, J. A., Carroll, P. J. & Schelter, E. J. Rearrangement in a tripodal nitroxide ligand to modulate the reactivity of a Ti–F bond. Inorg. Chem. 54, 9588–9593 (2015).
Mulks, F. F., Antoni, P. W., Rominger, F. & Hashmi, A. S. K. Cyclopropenylgold(I) complexes as aurated carbenoids or quasi-carbenes. Adv. Synth. Catal. 360, 1810–1821 (2018).
Werner, H., Fluegel, R., Windmueller, B., Michenfelder, A. & Wolf, J. Synthesis and reactions of stable 16-electron osmium(0) complexes [OsCl(NO)(PR3)2] including the X-ray crystal structure of [OsCl2(NO)(η1-CH=C=CPh2)(P-i-Pr3)2]. Organometallics 14, 612–618 (1995).
Schafer, D. F. II, Wolczanski, P. T. & Lobkovsky, E. B. Alkane binding implicated in reactions of (tBu3SiN=)3WHK and alkyl halides. Organometallics 30, 6518–6538 (2011).
Canovese, L. et al. Oxidative addition of allyl and propargyl halides on palladium(0) complexes bearing bidentate ligands with quinolinic structure. J. Organomet. Chem. 786, 21–30 (2015).
Semproni, S. P. & Chirik, P. J. N–H and N–C bond formation with an N2-derived dihafnium μ-nitrido complex. Organometallics 33, 3727–3737 (2014).
Chen, J.-T., Chen, Y.-K., Chu, J.-B., Lee, G.-H. & Wang, Y. Coordination of aniline to an (η1-allenyl)iridium complex leading to hydroanilination. Organometallics 16, 1476–1483 (1997).
Sperling, J. M. et al. Compression of curium pyrrolidine-dithiocarbamate enhances covalency. Nature 583, 396–399 (2020).
Kawano, M. et al. Structure analysis of photo-induced triplet phenylnitrene using synchrotron radiation. Chem. Lett. 32, 922–923 (2003).
Das, A., Chen, Y.-S., Reibenspies, J. H. & Powers, D. C. Characterization of a reactive Rh2 nitrenoid by crystalline matrix isolation. J. Am. Chem. Soc. 141, 16232–16236 (2019).
Williams, U. J. et al. Single crystal to single crystal transformation and hydrogen-atom transfer upon oxidation of a cerium coordination compound. Inorg. Chem. 52, 4142–4144 (2013).
Royle, C. G. et al. Single-crystal to single-crystal addition of H2 to [Ir(iPr-PONOP)(propene)][BArF4] and comparison between solid-state and solution reactivity. Organometallics 41, 3270–3280 (2022).
Sun, J. et al. A platinum(II) metallonitrene with a triplet ground state. Nat. Chem. 12, 1054–1059 (2020).
Reid, K. A. & Powers, D. C. In crystallo organometallic chemistry. Chem. Commun. 57, 4993–5003 (2021).
Fernandez-Bartolome, E., Martinez-Martinez, A., Resines-Urien, E., Piñeiro-Lopez, L. & Costa, J. S. Reversible single-crystal-to-single-crystal transformations in coordination compounds induced by external stimuli. Coord. Chem. Rev. 452, 214281 (2022).
Johnson, C. L. et al. A gold(I)–acetylene complex synthesised using single-crystal reactivity. Angew. Chem. Int. Ed. 63, e202404264 (2024).
Ting, P.-C., Lin, Y.-C., Lee, G.-H., Cheng, M.-C. & Wang, Y. Cyclopropenation and related reactions of ruthenium vinylidene complexes. J. Am. Chem. Soc. 118, 6433–6444 (1996).
Lo, Y.-H., Lin, Y.-C., Lee, G.-H. & Wang, Y. Synthesis and reactivity of the ruthenium cyclopropenyl complex with a Tp ligand. Organometallics 18, 982–988 (1999).
Zhang, X. et al. Fluoroalkyl N-triftosylhydrazones as easily decomposable diazo surrogates for asymmetric [2 + 1] cycloaddition: synthesis of chiral fluoroalkyl cyclopropenes and cyclopropanes. ACS Catal. 11, 8527–8537 (2021).
Becke, A. D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993).
Becke, A. D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 38, 3098–3100 (1988).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle–Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Staroverov, V. N., Scuseria, G. E., Tao, J. & Perdew, J. P. Comparative assessment of a new nonempirical density functional: molecules and hydrogen-bonded complexes. J. Chem. Phys. 119, 12129–12137 (2003).
Pandey, P. et al. Synthesis, electrochemical, and computational studies of organocerium(III) complexes with Ce–aryl sigma bonds. Organometallics 42, 1267–1277 (2023).
McCarver, G. A., Hinde, R. J. & Vogiatzis, K. D. Selecting quantum-chemical methods for lanthanide-containing molecules: a balance between accuracy and efficiency. Inorg. Chem. 59, 10492–10500 (2020).
Guillaumont, D. Quantum chemistry study of actinide(III) and lanthanide(III) complexes with tridentate nitrogen ligands. J. Phys. Chem. A 108, 6893–6900 (2004).
Strassner, T. & Taige, M. A. Evaluation of functionals O3LYP, KMLYP, and MPW1K in comparison to B3LYP for selected transition-metal compounds. J. Chem. Theory Comput. 1, 848–855 (2005).
Walsh, R. The cyclopropene pyrolysis story. Chem. Soc. Rev. 34, 714–732 (2005).
Acknowledgements
E.J.S. thanks the National Science Foundation (CHE-1955724) for the support of this work. We also thank the Jasco Center at the University of Pennsylvania for providing access to Raman spectrometry resources. J.A. acknowledges support for the theoretical component of this study by the US Department of Energy, Office of Science, Basic Energy Sciences, award DE-SC0020169. We thank the Center for Computational Research (CCR) at the University of Buffalo for providing computational resources. T.W.H. thanks the US Department of Energy, Office of Basic Energy Sciences, Chemical Sciences, Biosciences, and Geosciences Division under Contract DE-SC0001861. Work at Lawrence Berkeley National Laboratory was supported by the Director, Office of Science, Office of Basic Energy Sciences, Division of Chemical Sciences, Geosciences, and Biosciences Heavy Element Chemistry Program of the US Department of Energy (DOE) at LBNL under contract no. DE-AC02-05CH11231 (to S.G.M.). XANES measurements were performed at beamline 11-2 at the Stanford Synchrotron Radiation Lightsource, which is supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences under contract no. DE-AC02-76SF00515 (to S.G.M.).
Author information
Authors and Affiliations
Contributions
E.J.S., T.W.H., J.A. and S.G.M. conceived and supervised the study. X.Y. performed all calculations and created the associated figures and tables. B.D.V., G.T.K., O.O. and S.P. synthesized the starting materials. B.D.V. and S.P. designed and optimized the synthetic protocols and purification conditions for members of the M-0, M-1 and Ce-2 complexes. P.P. and H.G. collected the elemental analysis data. H.G. collected the Raman spectra and B.D.V. created the associated plots. B.D.V. collected the NMR spectra, produced the spectroscopic assignments, created the associated figures and wrote the associated discussion sections. P.W.S. collected the XANES spectra, created optimized fits, provided interpretations and created the associated figures. B.D.V. collected the electrochemical data, infrared spectra, electronic absorption spectra and created the associated figures. M.R.G., T.K. and A.M.B. collected the SC-XRD data and solved the structures whereas B.D.V. created the associated figures and video. B.D.V. and X.Y. wrote the manuscript with critical input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Thomas Albrecht-Schoenzart and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Plot of the occupancies of Ce-1∙C6H5CH3 (blue) and Ce-2∙C6H5CH3 (orange) versus time in a single crystal at 263 K.
Each data point represents the average occupancy over the collection of a complete single-crystal data set (99 min). Error bars represent the estimated standard deviations (esds) in the occupancies associated with each collection. The derived rate constant for the forward isomerization is 7.07 × 10−5 % ∙ min−1.
Extended Data Fig. 2 Infrared spectrum of Ce-1∙C6H5CH3 (NUJOL).
The spectrum was collected on crystals stored in the dark at 253 K for 3 days.
Extended Data Fig. 3 Infrared spectrum of Ce-1∙C6H5CH3 (NUJOL).
The spectrum was collected on crystals stored in the dark at 253 K for 10 days.
Extended Data Fig. 4 Infrared spectrum of Ce-1∙C6H5CH3 (NUJOL).
The spectrum was collected on crystals stored in the dark at 253 K for 121 days.
Extended Data Fig. 5 Experimental (blue) versus predicted (red) IR spectrum of Ce-1∙C6H5CH3 (NUJOL).
The spectrum was collected on crystals grown and stored in the dark at 243 K for 5 days.
Extended Data Fig. 6 Experimental (blue) versus predicted (red) IR spectrum of Ce-2∙C6H5CH3 (NUJOL).
The spectrum was collected on crystals grown and stored in the dark at 253 K for 30 days.
Extended Data Fig. 7 1H NMR (CD2Cl2, 500 MHz) specta of Ce-1.
Spectra were collected 300 K (top), 270 K (middle), and 230 K (bottom).
Supplementary information
Supplementary Information
Supplementary synthetic and characterization details, Discussions 1–3, Figs. 1–117 and Tables 1–26.
Supplementary Video 1
Thermal ellipsoid plots showing the SCSC transformation of Ce-1 to Ce-2.
Supplementary Data 1
Crystallographic data including structure factors for Th-1∙C6H5CH3. CCDC 2355964.
Supplementary Data 2
Crystallographic data including structure factors for Ti-1. CCDC 2355969.
Supplementary Data 3
Crystallographic data including structure factors for Zr-0. CCDC 2355962.
Supplementary Data 4
Crystallographic data including structure factors for Zr-1∙C6H5CH3. CCDC 2355961.
Supplementary Data 5
Crystallographic data including structure factors for Ce-1∙C6H5CH3. CCDC 2355965.
Supplementary Data 6
Crystallographic data including structure factors for Ce-1∙C6H5F. CCDC 2355960.
Supplementary Data 7
Crystallographic data including structure factors for Ce-1∙C6H6. CCDC 2355963.
Supplementary Data 8
Crystallographic data including structure factors for Ce-1∙Mes. CCDC 2355966.
Supplementary Data 9
Crystallographic data including structure factors for Ce-2∙C6H5CH3. CCDC 2355968.
Supplementary Data 10
Crystallographic data including structure factors for Hf-1∙C5H12. CCDC 2355967.
Supplementary Data 11
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 49.5 min over a ~99 min collection. CCDC 2357992.
Supplementary Data 12
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 215.5 min over a ~99 min collection. CCDC 2357993.
Supplementary Data 13
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 568.5 min over a ~99 min collection. CCDC 2357994.
Supplementary Data 14
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 913.5 min over a ~99 min collection. CCDC 2357995.
Supplementary Data 15
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 1,263.5 min over a ~99 min collection. CCDC 2357996.
Supplementary Data 16
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 1,644.5 min over a ~99 min collection. CCDC 2357997.
Supplementary Data 17
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 2,009.5 min over a ~99 min collection. CCDC 2357998.
Supplementary Data 18
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 2,353.5 min over a ~99 min collection. CCDC 2357999.
Supplementary Data 19
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 2,715.5 min over a ~99 min collection. CCDC 2358000.
Supplementary Data 20
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 3,089.5 min over a ~99 min collection. CCDC 2358001.
Supplementary Data 21
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 3,459.5 min over a ~99 min collection. CCDC 2358002.
Supplementary Data 22
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 3,834.5 min over a ~99 min collection. CCDC 2358003.
Supplementary Data 23
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 4,206.5 min over a ~99 min collection. CCDC 2358004.
Supplementary Data 24
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 4,567.5 min over a ~99 min collection. CCDC 2358005.
Supplementary Data 25
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 4,943.5 min over a ~99 min collection. CCDC 2358006.
Supplementary Data 26
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 5,321.5 min over a ~99 min collection. CCDC 2358007.
Supplementary Data 27
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 5,684.5 min over a ~99 min collection. CCDC 2358008.
Supplementary Data 28
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 6,040.5 min over a ~99 min collection. CCDC 2358009.
Supplementary Data 29
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 6,408.5 min over a ~99 min collection. CCDC 2358010.
Supplementary Data 30
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 6,771.5 min over a ~99 min collection. CCDC 2358011.
Supplementary Data 31
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 7,133.5 min over a ~99 min collection. CCDC 2358012.
Supplementary Data 32
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 7,491.5 min over a ~99 min collection. CCDC 2358013.
Supplementary Data 33
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 7,845.5 min over a ~99 min collection. CCDC 2358014.
Supplementary Data 34
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 8,203.5 min over a ~99 min collection. CCDC 2358015.
Supplementary Data 35
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 8,565.5 min over a ~99 min collection. CCDC 2358016.
Supplementary Data 36
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 8,910.5 min over a ~99 min collection. CCDC 2358017.
Supplementary Data 37
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 9,266.5 min over a ~99 min collection. CCDC 2358018.
Supplementary Data 38
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 9,637.5 min over a ~99 min collection. CCDC 2358019.
Supplementary Data 39
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 10,025.5 min over a ~99 min collection. CCDC 2358020.
Supplementary Data 40
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 10,401.5 min over a ~99 min collection. CCDC 2358021.
Supplementary Data 41
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 10,772.5 min over a ~99 min collection. CCDC 2358022.
Supplementary Data 42
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 11,161.5 min over a ~99 min collection. CCDC 2358023.
Supplementary Data 43
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 11,528.5 min over a ~99 min collection. CCDC 2358024.
Supplementary Data 44
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 11,894.5 min over a ~99 min collection. CCDC 2358025.
Supplementary Data 45
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 12,265.5 min over a ~99 min collection. CCDC 2358026.
Supplementary Data 46
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 12,643.5 min over a ~99 min collection. CCDC 2358027.
Supplementary Data 47
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 13,013.5 min over a ~99 min collection. CCDC 2358028.
Supplementary Data 48
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 13,385.5 min over a ~99 min collection. CCDC 2358029.
Supplementary Data 49
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 13,751.5 min over a ~99 min collection. CCDC 2358030.
Supplementary Data 50
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 14,104.5 min over a ~99 min collection. CCDC 2358031.
Supplementary Data 51
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 14,470.5 min over a ~99 min collection. CCDC 2358032.
Supplementary Data 52
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 14,821.5 min over a ~99 min collection. CCDC 2358033.
Supplementary Data 53
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 15,180.5 min over a ~99 min collection. CCDC 2358034.
Supplementary Data 54
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 15,524.5 min over a ~99 min collection. CCDC 2358035.
Supplementary Data 55
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 15,874.5 min over a ~99 min collection. CCDC 2358036.
Supplementary Data 56
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 16,227.5 min over a ~99 min collection. CCDC 2358037.
Supplementary Data 57
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 16,579.5 min over a ~99 min collection. CCDC 2358038.
Supplementary Data 58
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 16,932.5 min over a ~99 min collection. CCDC 2358039.
Supplementary Data 59
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 17,284.5 min over a ~99 min collection. CCDC 2358040.
Supplementary Data 60
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 17,635.5 min over a ~99 min collection. CCDC 2358041.
Supplementary Data 61
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 17,988.5 min over a ~99 min collection. CCDC 2358042.
Supplementary Data 62
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 18,343.5 min over a ~99 min collection. CCDC 2358043.
Supplementary Data 63
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 18,695.5 min over a ~99 min collection. CCDC 2358044.
Supplementary Data 64
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 18,796.5 min over a ~99 min collection. CCDC 2358045.
Supplementary Data 65
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 19,141.5 min over a ~99 min collection. CCDC 2358046.
Supplementary Data 66
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 19,496.5 min over a ~99 min collection. CCDC 2358047.
Supplementary Data 67
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 19,855.5 min over a ~99 min collection. CCDC 2358048.
Supplementary Data 68
Crystallographic data including structure factors for Ce-1∙C6H5CH3 at 263 K. Represents an average structure at 20,207.5 min over a ~99 min collection. CCDC 2358049.
Supplementary Data 69
Crystallographic tables for the SCSC reaction.
Source data
Source Data Fig. 1
Underlying data for the plot of the Ce-1 and Ce-2 occupancies versus time obtained via sequential SC-XRD collections at 263 K (Fig. 1b).
Source Data Fig. 2
Optimized xyz coordinates and stationary points for the Gibbs energy profiles for ring-opening isomerization of M-1 (M = Th, Ce, Hf, Zr, Ti) (Fig. 2b).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vincenzini, B.D., Yu, X., Paloc, S. et al. 4f-orbital covalency enables a single-crystal-to-single-crystal ring-opening isomerization in a CeIV–cyclopropenyl complex. Nat. Chem. 17, 961–967 (2025). https://doi.org/10.1038/s41557-025-01791-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01791-2