Abstract
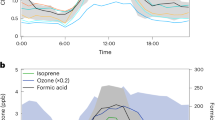
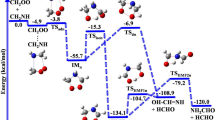
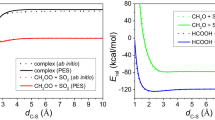
Criegee intermediates are highly reactive species that play a pivotal role in the chemistry of the atmosphere, substantially impacting global climate and air quality. They are formed through the reaction of ozone with alkenes and considerably influence the formation of hydroxyl radicals and aerosols through their unimolecular decomposition and their reaction with key atmospheric components, respectively. However, their interaction with water vapour, a major atmospheric component, remains inadequately characterized. Here, using both time-dependent laser-induced fluorescence experiments and full-dimensional dynamics calculations, we investigate the reaction of syn-CH3CHOO, a prevalent Criegee intermediate, with water vapour. Our results reveal a much higher reaction rate than previously estimated, challenging the conventional notion that unimolecular decomposition dominates syn-CH3CHOO removal. Notably, we uncover a complex mechanism involving a roaming process that enhances reactivity. Our findings necessitate a revised assessment of reactions involving syn-mono- and di-substituted Criegee intermediates with water, which are crucial for accurately estimating the OH budget derived from these intermediates.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data supporting the findings of this study can be accessed at figshare at https://doi.org/10.6084/m9.figshare.28630718.v1 (ref. 61). Source data are provided with this paper.
References
Criegee, R. Mechanism of ozonolysis. Angew. Chem. Int. Ed. 14, 745–752 (1975).
Stone, D., Whalley, L. K. & Heard, D. E. Tropospheric OH and HO2 radicals: field measurements and model comparisons. Chem. Soc. Rev. 41, 6348–6404 (2012).
Finlayson-Pitts, B. J. & Pitts Jr, J. N. Chemistry of the Upper and Lower Atmosphere: Theory, Experiments and Applications (Elsevier, 2000).
Heard, D. et al. High levels of the hydroxyl radical in the winter urban troposphere. Geophys. Res. Lett. 31, L18112 (2004).
Emmerson, K. & Carslaw, N. Night-time radical chemistry during the TORCH campaign. Atmos. Environ. 43, 3220–3226 (2009).
Cox, R. A. et al. Evaluated kinetic and photochemical data for atmospheric chemistry: volume VII—Criegee intermediates. Atmos. Chem. Phys. 20, 13497–13519 (2020).
Percival, C. J. et al. Regional and global impacts of Criegee intermediates on atmospheric sulphuric acid concentrations and first steps of aerosol formation. Faraday Discuss. 165, 45–73 (2013).
Caravan, R. et al. Observational evidence for Criegee intermediate oligomerization reactions relevant to aerosol formation in the troposphere. Nat. Geosci. 17, 219–226 (2024).
Chhantyal-Pun, R. et al. Criegee intermediate reactions with carboxylic acids: a potential source of secondary organic aerosol in the atmosphere. ACS Earth Space Chem. 2, 833–842 (2018).
Mauldin, R. L. et al. A new atmospherically relevant oxidant of sulphur dioxide. Nature 488, 193–196 (2012).
Calvert, J. G. et al. Chemical mechanisms of acid generation in the troposphere. Nature 317, 27–35 (1985).
Khan, M., Percival, C., Caravan, R., Taatjes, C. & Shallcross, D. Criegee intermediates and their impacts on the troposphere. Environ. Sci. Process. Impacts 20, 437–453 (2018).
Welz, O. et al. Direct kinetic measurements of Criegee intermediate (CH2OO) formed by reaction of CH2I with O2. Science 335, 204–207 (2012).
Shallcross, D. E., Khan, M. A. H., Taatjes, C. A. & Percival, C. J. New Insights Into the Role of Stabilized Criegee Intermediates in Tropospheric Chemistry from Direct Laboratory Studies (World Scientific, 2019).
Osborn, D. L. & Taatjes, C. A. The physical chemistry of Criegee intermediates in the gas phase. Int. Rev. Phys. Chem. 34, 309–360 (2015).
Taatjes, C. A. Criegee intermediates: what direct production and detection can teach us about reactions of carbonyl oxides. Annu. Rev. Phys. Chem. 68, 183–207 (2017).
Lester, M. I. & Klippenstein, S. J. Unimolecular decay of criegee intermediates to OH radical products: prompt and thermal decay processes. Acc. Chem. Res. 51, 978–985 (2018).
Chhantyal-Pun, R. et al. Criegee intermediates: production, detection and reactivity. Int. Rev. Phys. Chem. 39, 383–422 (2020).
Cabezas, C., Nakajima, M. & Endo, Y. Criegee intermediates meet rotational spectroscopy. Int. Rev. Phys. Chem. 39, 351–384 (2020).
Su, Y. T. et al. Extremely rapid self-reaction of the simplest Criegee intermediate CH2OO and its implications in atmospheric chemistry. Nat. Chem. 6, 477–483 (2014).
Long, B., Bao, J. L. & Truhlar, D. G. Rapid unimolecular reaction of stabilized Criegee intermediates and implications for atmospheric chemistry. Nat. Commun. 10, 2003 (2019).
Kidwell, N. M., Li, H. W., Wang, X. H., Bowman, J. M. & Lester, M. I. Unimolecular dissociation dynamics of vibrationally activated CH3CHOO Criegee intermediates to OH radical products. Nat. Chem. 8, 509–514 (2016).
Lin, H. Y. et al. Infrared identification of the Criegee intermediates syn- and anti-CH3CHOO, and their distinct conformation-dependent reactivity. Nat. Commun. 6, 7012 (2015).
Shabin, M., Kumar, A., Hakkim, H., Rudich, Y. & Sinha, V. Sources, sinks, and chemistry of stabilized Criegee intermediates in the Indo-Gangetic Plain. Sci. Total Environ. 896, 165281 (2023).
Zhou, X., Liu, Y., Dong, W. & Yang, X. Unimolecular reaction rate measurement of syn-CH3CHOO. J. Phys. Chem. Lett. 10, 4817–4821 (2019).
Li, Y.-L., Kuo, M.-T. & Lin, J. J.-M. Unimolecular decomposition rates of a methyl-substituted Criegee intermediate syn-CH3CHOO. RSC Adv. 10, 8518–8524 (2020).
Stephenson, T. A. & Lester, M. I. Unimolecular decay dynamics of Criegee intermediates: energy-resolved rates, thermal rates, and their atmospheric impact. Int. Rev. Phys. Chem. 39, 1–33 (2020).
Sheps, L., Scully, A. M. & Au, K. UV absorption probing of the conformer-dependent reactivity of a Criegee intermediate CH3CHOO. Phys. Chem. Chem. Phys. 16, 26701–26706 (2014).
Taatjes, C. A. et al. Direct measurements of conformer-dependent reactivity of the Criegee intermediate CH3CHOO. Science 340, 177–180 (2013).
Anglada, J. M. & Sole, A. Impact of the water dimer on the atmospheric reactivity of carbonyl oxides. Phys. Chem. Chem. Phys. 18, 17698–17712 (2016).
Lin, L. C. et al. Competition between H2O and (H2O)2 reactions with CH2OO/CH3CHOO. Phys. Chem. Chem. Phys. 18, 4557–4568 (2016).
Kuwata, K. T., Hermes, M. R., Carlson, M. J. & Zogg, C. K. Computational studies of the isomerization and hydration reactions of acetaldehyde oxide and methyl vinyl carbonyl oxide. J. Phys. Chem. A 114, 9192–9204 (2010).
Long, B., Bao, J. L. & Truhlar, D. G. Atmospheric chemistry of Criegee intermediates: unimolecular reactions and reactions with water. J. Am. Chem. Soc. 138, 14409–14422 (2016).
Ryzhkov, A. B. & Ariya, P. A. A theoretical study of the reactions of parent and substituted Criegee intermediates with water and the water dimer. Phys. Chem. Chem. Phys. 6, 5042–5050 (2004).
Chao, W., Hsieh, J. T., Chang, C. H. & Lin, J. J. Atmospheric chemistry. Direct kinetic measurement of the reaction of the simplest Criegee intermediate with water vapor. Science 347, 751–754 (2015).
Liu, F., Beames, J. M., Petit, A. S., McCoy, A. B. & Lester, M. I. Infrared-driven unimolecular reaction of CH3CHOO Criegee intermediates to OH radical products. Science 345, 1596–1598 (2014).
Vereecken, L., Glowacki, D. R. & Pilling, M. J. Theoretical chemical kinetics in tropospheric chemistry: methodologies and applications. Chem. Rev. 115, 4063–4114 (2015).
Lin, L. C., Chao, W., Chang, C. H., Takahashi, K. & Lin, J. J. Temperature dependence of the reaction of anti-CH3CHOO with water vapor. Phys. Chem. Chem. Phys. 18, 28189–28197 (2016).
Liu, Y. et al. A kinetic study of the CH2OO Criegee intermediate reaction with SO2, (H2O)2, CH2I2 and I atoms using OH laser induced fluorescence. Phys. Chem. Chem. Phys. 19, 20786–20794 (2017).
Yin, C. & Takahashi, K. Effect of unsaturated substituents in the reaction of Criegee intermediates with water vapor. Phys. Chem. Chem. Phys. 20, 20217–20227 (2018).
Chen, R., Shao, K., Fu, B. & Zhang, D. H. Fitting potential energy surfaces with fundamental invariant neural network. II. Generating fundamental invariants for molecular systems with up to ten atoms. J. Chem. Phys. 152, 204307 (2020).
Fu, B. & Zhang, D. H. Accurate fundamental invariant-neural network representation of ab initio potential energy surfaces. Natl Sci. Rev. 10, nwad321 (2023).
Mikosch, J. et al. Imaging nucleophilic substitution dynamics. Science 319, 183–186 (2008).
Townsend, D. et al. The roaming atom: straying from the reaction path in formaldehyde decomposition. Science 306, 1158–1161 (2004).
Li, Z. et al. Roaming in highly excited states: the central atom elimination of triatomic molecule decomposition. Science 383, 746–750 (2024).
Welz, O. et al. Rate coefficients of C1 and C2 Criegee intermediate reactions with formic and acetic acid near the collision limit: direct kinetics measurements and atmospheric implications. Angew. Chem. Int. Ed. 53, 4547–4550 (2014).
Greenwald, E. E., North, S. W., Georgievskii, Y. & Klippenstein, S. J. A two transition state model for radical–molecule reactions: applications to isomeric branching in the OH− isoprene reaction. J. Phys. Chem. A 111, 5582–5592 (2007).
Greenwald, E. E., North, S. W., Georgievskii, Y. & Klippenstein, S. J. A two transition state model for radical−molecule reactions: a case study of the addition of OH to C2H4. J. Phys. Chem. A 109, 6031–6044 (2005).
Anglada, J. M., Aplincourt, P., Bofill, J. M. & Cremer, D. Atmospheric formation of OH radicals and H2O2 from alkene ozonolysis under humid conditions. ChemPhysChem 3, 215–221 (2002).
Anglada, J. M., Gonzalez, J. & Torrent-Sucarrat, M. Effects of the substituents on the reactivity of carbonyl oxides. A theoretical study on the reaction of substituted carbonyl oxides with water. Phys. Chem. Chem. Phys. 13, 13034–13045 (2011).
Novelli, A., Vereecken, L., Lelieveld, J. & Harder, H. Direct observation of OH formation from stabilised Criegee intermediates. Phys. Chem. Chem. Phys. 16, 19941–19951 (2014).
1stOpt version 7.0 (7D-Soft High Technology, 2016).
Schmitt, G. & Comes, F. Photolysis of CH2I2 and 1,1-C2H4I2 at 300 nm. J. Photochem. 14, 107–123 (1980).
Barker, J. R. et al. Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation No. 17 JPL 10-6 (NASA, 2011); http://jpldataeval.jpl.nasa.gov
Knizia, G., Adler, T. B. & Werner, H. J. Simplified CCSD(T)-F12 methods: theory and benchmarks. J. Chem. Phys. 130, 054104 (2009).
Zhang, D. H. & Guo, H. Recent advances in quantum dynamics of bimolecular reactions. Annu. Rev. Phys. Chem. 67, 135–158 (2016).
Suleimanov, Y. V., Aoiz, F. J. & Guo, H. Chemical reaction rate coefficients from ring polymer molecular dynamics: theory and practical applications. J. Phys. Chem. A 120, 8488–8502 (2016).
Bowman, J. M. & Schatz, G. C. Theoretical studies of polyatomic bimolecular reaction dynamics. Annu. Rev. Phys. Chem. 46, 169–196 (1995).
Bao, J. L. & Truhlar, D. G. Variational transition state theory: theoretical framework and recent developments. Chem. Soc. Rev. 46, 7548–7596 (2017).
Fu, B. et al. Intersystem crossing and dynamics in O(3P) + C2H4 multichannel reaction: experiment validates theory. Proc. Natl Acad. Sci. USA 109, 9733–9738 (2012).
Liu, Y. et al. Reactivity of syn-CH3CHOO with H2O enhanced through a roaming mechanism in the entrance channel. figshare https://doi.org/10.6084/m9.figshare.28630718.v1 (2025).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos. 22288201 (D.H.Z.), 22173099 (B.F.), 21873098 (W.D.) and 22203092 (Y.F.)); the Scientific Instrument Developing Project of the Chinese Academy of Sciences (grant no. GJJSTD20220001 to X.Y.); the Strategic Priority Research Program of the Chinese Academy of Sciences (grant no. XDB0970203 to B.F.), the Innovation Program for Quantum Science and Technology (grant no. 2021ZD0303305 to D.H.Z.), Guangdong Science and Technology Program (grant nos. 2019ZT08L455 and 2019JC01X091 to X.Y.) and the Shenzhen Science and Technology Program (grant no. ZDSYS20200421111001787 to X.Y.). We thank the staff members of the Free Radical Detection Station (31127.02.DCLS.FRDS) at the Dalian Coherent Light Source (31127.02.DCLS) for providing technical support during the experiment. We acknowledge Y. V. Suleymanov, D. Manolopoulos and W. Fang for insightful discussions about the possible ring-polymer molecular dynamics and instanton theory calculations.
Author information
Authors and Affiliations
Contributions
X.Y., D.H.Z., W.D. and B.F. conceived and supervised the research. Yi.L., H.J., Yu.L., X.Z., H.L., X.W. and W.D. performed the experiments. Yi.L., H.J., Yu.L., X.Z., H.L., X.W., W.D. and X.Y. performed the experimental data analysis and interpretation. L.L., Y.F., H.W. and B.F. performed the potential energy surface construction and dynamics simulations. L.L., Y.F., H.W., X.L., R.T.S., B.F. and D.H.Z. discussed the theoretical results and analysis. X.Y., W.D. and B.F. wrote the paper, with contributions from all authors. All authors contributed to discussions about the content of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Gabor Czako and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, Discussion and Tables 1–6.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, Y., Liu, L., Fu, Y. et al. Reactivity of syn-CH3CHOO with H2O enhanced through a roaming mechanism in the entrance channel. Nat. Chem. 17, 897–903 (2025). https://doi.org/10.1038/s41557-025-01798-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01798-9
This article is cited by
-
Ultrafast solvent-modulated roaming mechanism in bromoform revealed by femtosecond X-ray solution scattering
Nature Communications (2026)
-
Theoretical study on the formation of Criegee intermediates from ozonolysis of CH≡CCH2OH
Journal of Molecular Modeling (2026)