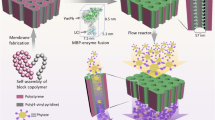
Abstract
Membranization of membraneless coacervates and condensates is emerging as a promising strategy to resolve their inherent susceptibility to fusion, ripening and environmental variations. Yet current membranization agents by design are largely limited to a subclass or a specific kind of coacervate or condensate systems. Here we develop a library of condensate-amphiphilic block polymers that can efficiently form a polymeric layer on the droplet interface for a wide spectrum of synthetic coacervates and biomolecular condensates. Condensate-amphiphilic block polymers are designed with a condenophilic block firmly anchored to the condensed phase, a condenophobic block extended to the dilute phase and a self-association block to promote membrane formation. Critical to our design is the condenophilic block of phenylboronic acid and amidoamine that target the disparate chemistry of condensed droplets via multivalent affinities. The condensate-amphiphilic block polymer membranes render the droplets mechanically robust against fusion, regulate interfacial properties such as permeability and stiffness, and substantially improve droplet tolerance to challenging conditions of temperature, salinity, pH and organic solvents.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. Source data can be found in files associated with this paper and in the figshare repository at https://doi.org/10.6084/m9.figshare.28069118 (ref. 72). All the data are also available from the corresponding author on request. Source data are provided with this paper.
References
Spruijt, E. Open questions on liquid–liquid phase separation. Commun. Chem. 6, 23 (2023).
Booij, H. L. & Bungenberg de Jong, H. G. Biocolloids and Their Interactions (Springer, 1956).
Lin, Z., Beneyton, T., Baret, J. C. & Martin, N. Coacervate droplets for synthetic cells. Small Methods 7, e2300496 (2023).
Brangwynne, C. P. et al. Germline P granules are liquid droplets that localize by controlled dissolution/condensation. Science 324, 1729–1732 (2009).
Banani, S. F., Lee, H. O., Hyman, A. A. & Rosen, M. K. Biomolecular condensates: organizers of cellular biochemistry. Nat. Rev. Mol. Cell Biol. 18, 285–298 (2017).
Shin, Y. & Brangwynne, C. P. Liquid phase condensation in cell physiology and disease. Science 357, eaaf4382 (2017).
Yewdall, N. A., André, A. A. M., Lu, T. & Spruijt, E. Coacervates as models of membraneless organelles. Curr. Opin. Colloid Interface Sci. 52, 101416 (2021).
Xu, C., Martin, N., Li, M. & Mann, S. Living material assembly of bacteriogenic protocells. Nature 609, 1029–1037 (2022).
Timilsena, Y. P., Akanbi, T. O., Khalid, N., Adhikari, B. & Barrow, C. J. Complex coacervation: principles, mechanisms and applications in microencapsulation. Int. J. Biol. Macromol. 121, 1276–1286 (2019).
Su, Q., Mehta, S. & Zhang, J. Liquid–liquid phase separation: orchestrating cell signaling through time and space. Mol. Cell 81, 4137–4146 (2021).
Brangwynne, C. P., Tompa, P. & Pappu, R. V. Polymer physics of intracellular phase transitions. Nat. Phys. 11, 899–904 (2015).
Kilgore, H. R. & Young, R. A. Learning the chemical grammar of biomolecular condensates. Nat. Chem. Biol. 18, 1298–1306 (2022).
Yang, S. et al. Steering coacervation by a pair of broad-spectrum regulators. ACS Nano 13, 2420–2426 (2019).
Chen, X. et al. Optothermally programmable liquids with spatiotemporal precision and functional complexity. Adv. Mater. 34, e2205563 (2022).
Zhao, P. et al. Nanoparticle-assembled vacuolated coacervates control macromolecule spatiotemporal distribution to provide a stable segregated cell microenvironment. Adv. Mater. 33, e2007209 (2021).
Lu, T., Javed, S., Bonfio, C. & Spruijt, E. Interfacing coacervates with membranes: from artificial organelles and hybrid protocells to intracellular delivery. Small Methods 7, e2300294 (2023).
Gao, N. & Mann, S. Membranized coacervate microdroplets: from versatile protocell models to cytomimetic materials. Acc. Chem. Res. 56, 297–307 (2023).
Li, M., Harbron, R. L., Weaver, J. V., Binks, B. P. & Mann, S. Electrostatically gated membrane permeability in inorganic protocells. Nat. Chem. 5, 529–536 (2013).
Dora Tang, T. Y. et al. Fatty acid membrane assembly on coacervate microdroplets as a step towards a hybrid protocell model. Nat. Chem. 6, 527–533 (2014).
Mason, A. F., Buddingh, B. C., Williams, D. S. & van Hest, J. C. M. Hierarchical self-assembly of a copolymer-stabilized coacervate protocell. J. Am. Chem. Soc. 139, 17309–17312 (2017).
Zhao, C. et al. Membranization of coacervates into artificial phagocytes with predation toward bacteria. ACS Nano 15, 10048–10057 (2021).
Gao, S. & Srivastava, S. Comb polyelectrolytes stabilize complex coacervate microdroplet dispersions. ACS Macro Lett. 11, 902–909 (2022).
Li, Q. et al. A self-templated route to monodisperse complex droplets as artificial extremophile-mimic from coacervate-liposome interplay. Preprint at bioRxiv https://doi.org/10.1101/2021.02.19.432011 (2022).
Yin, C., Lin, Z., Jiang, X., Martin, N. & Tian, L. Engineering the coacervate microdroplet interface via polyelectrolyte and surfactant complexation. ACS Appl. Mater. Interfaces 15, 27447–27456 (2023).
Ji, Y., Lin, Y. & Qiao, Y. Plant cell-inspired membranization of coacervate protocells with a structured polysaccharide layer. J. Am. Chem. Soc. 145, 12576–12585 (2023).
Cuylen, S. et al. Ki-67 acts as a biological surfactant to disperse mitotic chromosomes. Nature 535, 308–312 (2016).
Simon, J. R., Carroll, N. J., Rubinstein, M., Chilkoti, A. & López, G. P. Programming molecular self-assembly of intrinsically disordered proteins containing sequences of low complexity. Nat. Chem. 9, 509–515 (2017).
Folkmann, A. W., Putnam, A., Lee, C. F. & Seydoux, G. Regulation of biomolecular condensates by interfacial protein clusters. Science 373, 1218–1224 (2021).
Kelley, F. M., Favetta, B., Regy, R. M., Mittal, J. & Schuster, B. S. Amphiphilic proteins coassemble into multiphasic condensates and act as biomolecular surfactants. Proc. Natl Acad. Sci. USA 118, e2109967118 (2021).
Tschurikow, X. et al. Amphiphiles formed from synthetic DNA-nanomotifs mimic the stepwise dispersal of transcriptional clusters in the cell nucleus. Nano Lett. 23, 7815–7824 (2023).
Thody, S. A. et al. Small-molecule properties define partitioning into biomolecular condensates. Nat. Chem. 16, 1794–1802 (2024).
Panganiban, B. et al. Random heteropolymers preserve protein function in foreign environments. Science 359, 1239–1243 (2018).
Ruan, Z. et al. Population-based heteropolymer design to mimic protein mixtures. Nature 615, 251–258 (2023).
Liu, C. et al. A boronic acid-rich dendrimer with robust and unprecedented efficiency for cytosolic protein delivery and CRISPR-Cas9 gene editing. Sci. Adv. 5, eaaw8922 (2019).
Santos, F. K. G., Neto, E. L. B., Moura, M. C. P., Dantas, T. N. C. & Neto, A. A. D. Molecular behavior of ionic and nonionic surfactants in saline medium. Colloids Surf. A 333, 156–162 (2009).
Taylor, N. O., Wei, M. T., Stone, H. A. & Brangwynne, C. P. Quantifying dynamics in phase-separated condensates using fluorescence recovery after photobleaching. Biophys. J. 117, 1285–1300 (2019).
Yewdall, N. A. et al. Physicochemical characterization of polymer-stabilized coacervate protocells. ChemBioChem 20, 2643–2652 (2019).
Lu, T. & Spruijt, E. Multiphase complex coacervate droplets. J. Am. Chem. Soc. 142, 2905–2914 (2020).
Rana, U. et al. Asymmetric oligomerization state and sequence patterning can tune multiphase condensate miscibility. Nat. Chem. 16, 1073–1082 (2024).
Huang, Y. et al. Encoding coacervate droplets with paramagnetism for dynamical reconfigurability and spatial addressability. ACS Nano 17, 6234–6246 (2023).
Kota, D., Prasad, R. & Zhou, H. X. Adenosine triphosphate mediates phase separation of disordered basic proteins by bridging intermolecular interaction networks. J. Am. Chem. Soc. 146, 1326–1336 (2024).
Eid, J., Greige-Gerges, H., Monticelli, L. & Jraij, A. Elastic moduli of lipid membranes: reproducibility of AFM measures. Chem. Phys. Lipids 234, 105011 (2021).
Riback, J. A. et al. Stress-triggered phase separation is an adaptive, evolutionarily tuned response. Cell 168, 1028–1040 (2017).
Yu, M. & Nishiumi, H. Theory of phase separation in mixtures with lower critical solution temperature. J. Phys. Chem. 96, 842–845 (1992).
Davis, C. R., Martinez, C. J., Howarter, J. A. & Erk, K. A. Diffusion-controlled spontaneous emulsification of water-soluble oils via micelle swelling. Langmuir 36, 7517–7527 (2020).
Sacanna, S., Kegel, W. K. & Philipse, A. P. Thermodynamically stable Pickering emulsions. Phys. Rev. Lett. 98, 158301 (2007).
Oron, A., Davis, S. H. & Bankoff, S. G. Long-scale evolution of thin liquid films. Mod. Phys. 69, 931 (1997).
Lee, J. & Babadagli, T. Comprehensive review on heavy-oil emulsions: colloid science and practical applications. Chem. Eng. Sci. 228, 115962 (2020).
Bates, F. S. & Fredrickson, G. H. Block copolymers—designer soft materials. Phys. Today 52, 32–38 (1999).
Heggestad, J. T., Fontes, C. M., Joh, D. Y., Hucknall, A. M. & Chilkoti, A. In pursuit of zero 2.0: recent developments in nonfouling polymer brushes for immunoassays. Adv. Mater. 32, e1903285 (2020).
Shen, J., Liu, G., Han, Y. & Jin, W. Artificial channels for confined mass transport at the sub-nanometre scale. Nat. Rev. Mater. 6, 294–312 (2021).
Sitarska, E. et al. Sensing their plasma membrane curvature allows migrating cells to circumvent obstacles. Nat. Commun. 14, 5644 (2023).
Estirado, E. M., Mason, A. F., Garcia, M. Á. A., van Hest, J. C. M. & Brunsveld, L. Supramolecular nanoscaffolds within cytomimetic protocells as signal localization hubs. J. Am. Chem. Soc. 142, 9106–9111 (2020).
Liu, S. et al. Enzyme-mediated nitric oxide production in vasoactive erythrocyte membrane-enclosed coacervate protocells. Nat. Chem. 12, 1165–1173 (2020).
Priftis, D. & Tirrell, M. Phase behaviour and complex coacervation of aqueous polypeptide solutions. Soft Matter 8, 9396–9405 (2012).
Baruch Leshem, A. et al. Biomolecular condensates formed by designer minimalistic peptides. Nat. Commun. 14, 421 (2023).
Bohidar, H., Dubin, P. L., Majhi, P. R., Tribet, C. & Jaeger, W. Effects of protein-polyelectrolyte affinity and polyelectrolyte molecular weight on dynamic properties of bovine serum albumin-poly(diallyldimethylammonium chloride) coacervates. Biomacromolecules 6, 1573–1585 (2005).
Chen, N., Zhao, Z., Wang, Y. & Dimova, R. Resolving the mechanisms of soy glycinin self-coacervation and hollow-condensate formation. ACS Macro Lett. 9, 1844–1852 (2020).
Schuster, B. S. et al. Controllable protein phase separation and modular recruitment to form responsive membraneless organelles. Nat. Commun. 9, 2985 (2018).
Mohanty, P. et al. A synergy between site-specific and transient interactions drives the phase separation of a disordered, low-complexity domain. Proc. Natl Acad. Sci. USA 120, e2305625120 (2023).
Patel, A. et al. A liquid-to-solid phase transition of the ALS protein FUS accelerated by disease mutation. Cell 162, 1066–1077 (2015).
Huang, Y. et al. Methylene blue accelerates liquid-to-gel transition of tau condensates impacting tau function and pathology. Nat. Commun. 14, 5444 (2023).
Mitrea, D. M. et al. Nucleophosmin integrates within the nucleolus via multi-modal interactions with proteins displaying R-rich linear motifs and rRNA. eLife 5, e13571 (2016).
Ye, S. et al. Micropolarity governs the structural organization of biomolecular condensates. Nat. Chem. Biol. 20, 443–451 (2024).
Volpe, C. D. & Siboni, S. The Wilhelmy method: a critical and practical review. Surf. Innov. 6, 120–132 (2018).
Ijavi, M. et al. Surface tensiometry of phase separated protein and polymer droplets by the sessile drop method. Soft Matter 17, 1655–1662 (2021).
Frey, S. et al. Surface properties determining passage rates of proteins through nuclear pores. Cell 174, 202–217.e9 (2018).
Yang, T. et al. Droplet-based microfluidic temperature-jump platform for the rapid assessment of biomolecular kinetics. Anal. Chem. 94, 16675–16684 (2022).
Villois, A. et al. Droplet microfluidics for the label-free extraction of complete phase diagrams and kinetics of liquid–liquid phase separation in finite volumes. Small 18, 2202606 (2022).
Beneyton, T., Love, C., Girault, M., Tang, T. Y. D. & Baret, J. C. High-throughput synthesis and screening of functional coacervates using microfluidics. ChemSystemsChem 2, e2000022 (2020).
Wang, W. et al. Highly robust crystalsome via directed polymer crystallization at curved liquid/liquid interface. Nat. Commun. 7, 10599 (2016).
Tang, D. et al. Universal membranization of synthetic coacervates and biomolecular condensates towards ultrastability and spontaneous emulsification. figshare https://doi.org/10.6084/m9.figshare.28069118 (2024).
Acknowledgements
L.J. is in debt to X. Zhang from Westlake University and X. Yang from South China University of Technology for their advice on protein condensates. L.J. acknowledges support by the National Natural Science Foundation of China (numbers 22122201 and 22272060), the CYGJ Program of Guangzhou (number 2024D03J0003), the State Key Laboratory of Pulp and Paper Engineering (numbers 2024QN09, 2024ZD01 and 2024ZD06), the TCL Science and Technology Innovation Fund (number x2fkE5240020), the Guangdong Basic and Applied Basic Research Foundation (number 2023A1515010956), the Fundamental Research Funds for the Central Universities (number 2024ZYGXZR017) and the Recruitment Program of Guangdong (number 2016ZT06C322).
Author information
Authors and Affiliations
Contributions
D.T. and L.J. conceived the project and designed the experiments. D.T. performed most of the experiments. J.Z. expressed and purified the proteins. Hao Wang conducted the FLIM experiments to measure the micropolarity and viscosity of condensed phases. N.C. provided the SG protein. Hui Wang suggested the monomer selection for protein affinity. Y.H. advised on the preparation of protein condensates. All the authors analysed the data and contributed to discussing the results and writing the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Additional methods, Supplementary Tables 1–7, Figs. 1–28 and Notes 1–4.
Supplementary Video 1
Brownian motion and anti-fusing dynamics of CAP-laden droplets (COND1@CAP15) in cell-sized confinement.
Supplementary Video 2
Collision events of closely packed membranized droplets (COND3@CAP15) do not lead to fusion.
Supplementary Video 3
Optical tweezers manipulate the collision and fusion of membraneless droplets (COND3).
Supplementary Video 4
Optical tweezers force the collision and deformation of the membranized droplets (COND3@CAP23).
Supplementary Video 5
Spontaneous emulsification observed by transmission channel (COND1@CAP15).
Supplementary Video 6
Spontaneous emulsification by fluorescence channel (COND1@CAP15).
Source Data for Supplementary Figures
Source data for Supplementary figures (this file is also uploaded to figshare 10.6084/m9.figshare.28069118).
Source data
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, D., Zhu, J., Wang, H. et al. Universal membranization of synthetic coacervates and biomolecular condensates towards ultrastability and spontaneous emulsification. Nat. Chem. 17, 911–923 (2025). https://doi.org/10.1038/s41557-025-01800-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01800-4
This article is cited by
-
Stabilizing condensates and coacervates all the same
Nature Reviews Materials (2025)
-
Study on Preparation of High-Quality Magnesium Hydroxide and Calcium Hydroxide Products from Dolomite
JOM (2025)