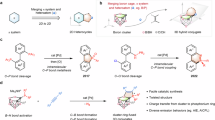
Abstract
Boracycles are important functional scaffolds, often exhibiting superior or unique performance compared with their carbon analogues. Five-membered oxaboracycles are key pharmacophores in Food and Drug Administration-approved boron drugs. Meanwhile, six-membered boron-doped polycyclic aromatic hydrocarbons enhance the diversification and functionality of molecular materials. However, boron-containing four-membered rings are less studied owing to limited preparative approaches. Their inherent ring strain makes their synthesis thermodynamically unfavourable and hinders the exploration of their properties and applications. Here we report a triplet energy transfer catalysis for crafting air-stable benzoboretenes through intramolecular coupling of boron–carbon-centred diradicals. In addition, by modulating substrate π-conjugation structures and excitation energies, boron–carbon-centred diradicals can undergo formal 1,6- and 1,5-cyclization to deliver dihydroborinine and dihydrocyclopropaborole derivatives, respectively. The metal-free neutral reaction conditions ensure a broad reaction scope, resulting in structurally diverse boracycles that are stable enough to be purified via column chromatography. Further modification of the boracycles enables the facile synthesis of oxaborabicycles and dihydroborinine-fused polycyclic aromatic hydrocarbons with unique optoelectronic properties.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2352027 (2), 2352030 (22S), 2352031 (55), 2352034 (56), 2352033 (62) and 2352035 (70). Copies of the data can be obtained free of charge at https://www.ccdc.cam.ac.uk/structures/.
References
McConnell, C. R. & Liu, S. Y. Late-stage functionalization of BN-heterocycles. Chem. Soc. Rev. 48, 3436–3453 (2019).
Su, Y. & Kinjo, R. Small molecule activation by boron-containing heterocycles. Chem. Soc. Rev. 48, 3613–3659 (2019).
Su, X. et al. 9-Borafluorenes: synthesis, properties, and reactivity. Chem. Rev. 121, 4147–4192 (2021).
Braunschweig, H., Chiu, C.-W., Radacki, K. & Kupfer, T. Synthesis and structure of a carbene-stabilized π-boryl anion. Angew. Chem. Int. Ed. 49, 2041–2044 (2010).
Tian, D., Jiang, J., Hu, H., Zhang, J. & Cui, C. One-step access to luminescent pentaaryldiazaboroles via C–C double bond formation from imidoylstannanes. J. Am. Chem. Soc. 134, 14666–14669 (2012).
Longobardi, L. E., Liu, L., Grimme, S. & Stephan, D. W. Stable borocyclic radicals via frustrated Lewis pair hydrogenations. J. Am. Chem. Soc. 138, 2500–2503 (2016).
Sun, Q. et al. Borole/borapyramidane relationship. J. Am. Chem. Soc. 144, 7815–7821 (2022).
Kirkpatrick, P. & Ellis, C. Chemical space. Nature 432, 823–823 (2004).
Dobson, C. M. Chemical space and biology. Nature 432, 824–828 (2004).
Grams, R. J. et al. The rise of boron-containing compounds: advancements in synthesis, medicinal chemistry, and emerging pharmacology. Chem. Rev. 124, 2441–2511 (2024).
Graham, B. J., Windsor, I. W., Gold, B. & Raines, R. T. Boronic acid with high oxidative stability and utility in biological contexts. Proc. Natl Acad. Sci. USA 118, e2013691118 (2021).
Lyu, H., Kevlishvili, I., Yu, X., Liu, P. & Dong, G. Boron insertion into alkyl ether bonds via zinc/nickel tandem catalysis. Science 372, 175–182 (2021).
Yuan, K. & Ingleson, M. J. Haloboration of o-alkynyl phenols generates halogenated bicyclic-boronates. Angew. Chem. Int. Ed. 62, e202301463 (2023).
Wood, T. K., Piers, W. E., Keay, B. A. & Parvez, M. 9-Boraanthracene derivatives stabilized by N-heterocyclic carbenes. Angew. Chem. Int. Ed. 48, 4009–4012 (2009).
Zhou, Z., Wakamiya, A., Kushida, T. & Yamaguchi, S. Planarized triarylboranes: stabilization by structural constraint and their plane-to-bowl conversion. J. Am. Chem. Soc. 134, 4529–4532 (2012).
Farrell, J. M. et al. Tunable low-LUMO boron-doped polycyclic aromatic hydrocarbons by general one-pot C–H borylations. J. Am. Chem. Soc. 141, 9096–9104 (2019).
Scholz, A. S. et al. BNB-doped phenalenyls: modular synthesis, optoelectronic properties, and one-electron reduction. J. Am. Chem. Soc. 142, 11072–11083 (2020).
Deng, C. L. et al. Air- and photo-stable luminescent carbodicarbene-azaboraacenium ions. Nat. Chem. 16, 437–445 (2024).
Su, W. et al. Copper-catalysed asymmetric hydroboration of alkenes with 1,2-benzazaborines to access chiral naphthalene isosteres. Nat. Chem. 16, 1312–1319 (2024).
Schacht, W. & Kaufmann, D. Erste Synthese eines Benzodihydroborets. J. Organomet. Chem. 339, 33–39 (1988).
Hergel, A., Pritzkow, H. & Siebert, W. Synthesis and reactivity of a naphtho[l,8‐bc]boret. Angew. Chem. Int. Ed. 33, 1247–1248 (1994).
Guven, Z. et al. Reactions of a four-membered borete with carbon, silicon, and gallium donor ligands: fused and spiro-type boracycles. Chem. Eur. J. 28, e202200673 (2022).
Pues, C., Baum, G., Massa, W. & Berndt, A. Structure of a homoborirene. Z. Naturforsch. 43b, 275–279 (1988).
Zhao, H. & Gabbaï, F. P. A bidentate Lewis acid with a telluronium ion as an anion-binding site. Nat. Chem. 2, 984–990 (2010).
Araneda, J. F., Neue, B., Piers, W. E. & Parvez, M. Photochemical synthesis of a ladder diborole: a new boron-containing conjugate material. Angew. Chem. Int. Ed. 51, 8546–8550 (2012).
Wang, J., Zhu, S., Ma, W., Lin, Z. & Ye, Q. BN-butafulvenes and their application in the synthesis of highly substituted BN-9,1-naphthalenes. Angew. Chem. Int. Ed. 62, e202219062 (2023).
Arif, A. M., Cowley, A. H., Pakulski, M. & Power, J. M. Diphospha- and diarsa-diboretanes. Four-membered rings containing boron and phosphorus or arsenic. J. Chem. Soc. Chem. Commun. 11, 889–890 (1986).
Edel, K. et al. The Dewar isomer of 1,2-dihydro-1,2-azaborinines: isolation, fragmentation, and energy storage. Angew. Chem. Int. Ed. 57, 5296–5300 (2018).
Ishida, Y., Donnadieu, B. & Bertrand, G. Stable four-π-electron, four-membered heterocyclic cations and carbenes. Proc. Natl Acad. Sci. USA 103, 13585–13588 (2006).
Ian, M. & Peter, P. Formation of the novel neopentylidenoborane Me3Si(tBu)N=B=CHtBu from the tantalum neopentylidene complex [(η-C5H5)Cl2Ta=CHtBu] by metathesis. J. Chem. Soc. Chem. Commun. 3, 183–185 (1988).
Ito, M., Tokitoh, N. & Okazaki, R. 1,3,2,4-Diselenastannaboretane, a novel selenium-containing four-membered boracycle: synthesis, structure and thermal cycloreversion into a selenoxoborane. Chem. Commun. 22, 2495–2496 (1998).
Leifert, D. & Studer, A. The persistent radical effect in organic synthesis. Angew. Chem. Int. Ed. 59, 74–108 (2020).
Parsaee, F. et al. Radical philicity and its role in selective organic transformations. Nat. Rev. Chem. 5, 486–499 (2021).
Chan, A. Y. et al. Metallaphotoredox: the merger of photoredox and transition metal catalysis. Chem. Rev. 122, 1485–1542 (2022).
Capaldo, L., Ravelli, D. & Fagnoni, M. Direct photocatalyzed hydrogen atom transfer (HAT) for aliphatic C–H bonds elaboration. Chem. Rev. 122, 1875–1924 (2022).
Golden, D. L., Suh, S.-E. & Stahl, S. S. Radical C(sp3)–H functionalization and cross-coupling reactions. Nat. Rev. Chem. 6, 405–427 (2022).
Parrish, J. D. & Little, R. D. Diradicals in synthesis. in Radicals in Organic Synthesis (eds Renaud, P. & Sibi, M. P.) 383–406 (Wiley-VCH, 2001).
Abe, M. Diradicals. Chem. Rev. 113, 7011–7088 (2013).
Hinz, A., Bresien, J., Breher, F. & Schulz, A. Heteroatom-based diradical(oid)s. Chem. Rev. 123, 10468–10526 (2023).
Kaim, W., Hosmane, N. S., Záliš, S., Maguire, J. A. & Lipscomb, W. N. Boron atoms as spin carriers in two- and three-dimensional systems. Angew. Chem. Int. Ed. 48, 5082–5091 (2009).
Maiti, A. et al. Anionic boron- and carbon-based hetero-diradicaloids spanned by a p-phenylene bridge. J. Am. Chem. Soc. 143, 3687–3692 (2021).
Walton, J. C. et al. EPR studies of the generation, structure, and reactivity of n-heterocyclic carbene borane radicals. J. Am. Chem. Soc. 132, 2350–2358 (2010).
Zhou, N., Yuan, X. A., Zhao, Y., Xie, J. & Zhu, C. Synergistic photoredox catalysis and organocatalysis for inverse hydroboration of imines. Angew. Chem. Int. Ed. 57, 3990–3994 (2018).
Xia, P. J. et al. Photoinduced single-electron transfer as an enabling principle in the radical borylation of alkenes with NHC-borane. Angew. Chem. Int. Ed. 59, 6706–6710 (2020).
Xu, W., Jiang, H., Leng, J., Ong, H. W. & Wu, J. Visible-light-induced selective defluoroborylation of polyfluoroarenes, gem-difluoroalkenes, and trifluoromethylalkenes. Angew. Chem. Int. Ed. 59, 4009–4016 (2020).
Shu, C., Noble, A. & Aggarwal, V. K. Metal-free photoinduced C(sp3)–H borylation of alkanes. Nature 586, 714–719 (2020).
Kim, J. H. et al. A radical approach for the selective C–H borylation of azines. Nature 595, 677–683 (2021).
Miao, Y.-Q. et al. A general photo-induced wide-scope regioselective hydroboration of alkenes without using a photocatalyst or an external initiator. Green Chem. 24, 7113–7121 (2022).
Peng, T. Y., Zhang, F. L. & Wang, Y. F. Lewis base-boryl radicals enabled borylation reactions and selective activation of carbon–heteroatom bonds. Acc. Chem. Res. 56, 169–186 (2023).
Tian, Y.-M., Guo, X.-N., Braunschweig, H., Radius, U. & Marder, T. B. Photoinduced borylation for the synthesis of organoboron compounds. Chem. Rev. 121, 3561–3597 (2021).
Grosskopf, J., Kratz, T., Rigotti, T. & Bach, T. Enantioselective photochemical reactions enabled by triplet energy transfer. Chem. Rev. 122, 1626–1653 (2022).
Molloy, J. J. et al. Boron-enabled geometric isomerization of alkenes via selective energy-transfer catalysis. Science 369, 302–306 (2020).
Kleinmans, R. et al. Intermolecular [2π+2σ]-photocycloaddition enabled by triplet energy transfer. Nature 605, 477–482 (2022).
Becker, M. R., Wearing, E. R. & Schindler, C. S. Synthesis of azetidines via visible-light-mediated intermolecular [2+2] photocycloadditions. Nat. Chem. 12, 898–905 (2020).
Blum, T. R., Miller, Z. D., Bates, D. M., Guzei, I. A. & Yoon, T. P. Enantioselective photochemistry through Lewis acid–catalyzed triplet energy transfer. Science 354, 1391–1395 (2016).
Doan, T. H. et al. Methylene bridging effect on the structures, Lewis acidities and optical properties of semi-planar triarylboranes. Chem. Eur.J. 27, 1736–1743 (2021).
Munster, N., Parker, N. A., van Dijk, L., Paton, R. S. & Smith, M. D. Visible light photocatalysis of 6π heterocyclization. Angew. Chem. Int. Ed. 56, 9468–9472 (2017).
Acknowledgements
We thank H. H.-Y. Sung and I. Williams for single-crystal X-ray diffraction analyses, and Y. Zuo for quantum efficiency measurement. We acknowledge the start-up funding (project no. R9804 to Y.Q.) from the Hong Kong University of Science and Technology (HKUST), start-up funding (project no. 4933621 to H.L.) from The Chinese University of Hong Kong (CUHK), Early Career Scheme from Research Grants Council of Hong Kong (project nos. 26307123 to Y.Q. and 24307423 to H.L.), General Research Fund from Research Grants Council of Hong Kong (project no. HKUST16300021 to Z.L.), Young Scholar Fund (project no. 22301254 to Y.Q.) from National Natural Science Foundation of China, and the Croucher Foundation for financial support.
Author information
Authors and Affiliations
Contributions
X.W., H.L. and Y.Q. conceived and designed the experiments. X.W., Z.Y. and W.S. performed experiments. P.Z. and Z.L. performed DFT calculations. X.W., P.Z., H.L., Z.L. and Y.Q. wrote the paper. H.L., Z.L. and Y.Q. directed the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–31, Supplementary Table 1–13, experimental details, computational details, data and spectra.
Supplementary Data 1
Crystallographic data for compound 2; CCDC reference 2352027.
Supplementary Data 2
Crystallographic data for compound 22S; CCDC reference 2352030.
Supplementary Data 3
Crystallographic data for compound 55; CCDC reference 2352031.
Supplementary Data 4
Crystallographic data for compound 56; CCDC reference 2352034.
Supplementary Data 5
Crystallographic data for compound 62; CCDC reference 2352033.
Supplementary Data 6
Crystallographic data for compound 70; CCDC reference 2352035.
Supplementary Data 7
The xyz coordinates of DFT calculation results.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Zhang, P., Yang, Z. et al. Synthesis of strained, air-stable boracycles via boron–carbon-centred diradicals. Nat. Chem. 17, 663–671 (2025). https://doi.org/10.1038/s41557-025-01807-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01807-x
This article is cited by
-
Bench-stable boracyclobutenes
Nature Chemistry (2025)