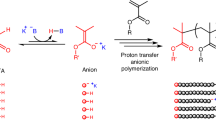
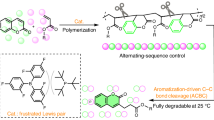
Abstract
Anionic polymerizations of vinyl monomers are powerful synthetic platforms for making well-defined materials. However, these reactions are extremely sensitive to moisture and oxygen, require the use of highly purified reagents, must be run at low temperatures, and use hazardous and difficult-to-handle alkyl lithium initiators. Together, these drawbacks limit the practicality of these polymerizations and impede their widespread usage. On this basis, the development of a user-friendly anionic polymerization process for methacrylates is a grand challenge. Here we report an anionic polymerization of methacrylates mediated by CO2 that can be run at elevated temperatures and uses an easy-to-handle solid initiator. The reversible addition of CO2 to the enolate chain end efficiently tempers the reactivity of the anion, giving polymers with narrow molar mass distributions and excellent molecular weight targeting at elevated temperatures. Our scalable and more user-friendly CO2-mediated method improves the accessibility and safety of anionic polymerizations and facilitates the production of a variety of polymeric materials.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All of the data that support the findings of this study are available in the main text or Supplementary Information. 1H and 13C NMR files and text files of GPC chromatograms, in situ CO2 evolution traces, and MALDI-TOF MS spectra have been deposited in eCommons (Cornell’s Digital Repository) at https://doi.org/10.7298/s733-8m37 (ref. 53). Source data are provided with this paper.
References
Ali, U., Karim, K. J. B. A. & Buang, N. A. A review of the properties and applications of poly (methyl methacrylate) (PMMA). Polym. Rev. 55, 678–705 (2015).
Rösler, A., Vandermeulen, G. W. M. & Klok, H.-A. Advanced drug delivery devices via self-assembly of amphiphilic block copolymers. Adv. Drug Deliv. Rev. 64, 270–279 (2012).
Perumal, S., Atchudan, R. & Lee, W. A review of polymeric micelles and their applications. Polymers 14, 2510 (2022).
Webster, O. W. The discovery and commercialization of group transfer polymerization. J. Polym. Sci. A 38, 2855–2860 (2000).
Destarac, M. Controlled radical polymerization: industrial stakes, obstacles and achievements. Macromol. React. Eng. 4, 165–179 (2010).
Truong, N. P., Jones, G. R., Bradford, K. G. E., Konkolewicz, D. & Anastasaki, A. A comparison of RAFT and ATRP methods for controlled radical polymerization. Nat. Rev. Chem. 5, 859–869 (2021).
Shanmugam, S. & Matyjaszewski, K. in Reversible Deactivation Radical Polymerization: Mechanisms and Synthetic Methodologies Vol. 1 (eds Matyjaszewski, K. et al.) 1–39 (American Chemical Society, 2018).
Ntetsikas, K., Ladelta, V., Bhaumik, S. & Hadjichristidis, N. Quo vadis carbanionic polymerization? ACS Poly. Au 3, 158–181 (2023).
Hirao, A., Goseki, R. & Ishizone, T. Advances in living anionic polymerization: from functional monomers, polymerization systems, to macromolecular architectures. Macromolecules 47, 1883–1905 (2014).
Hadjichristidis, N., Iatrou, H., Pitsikalis, M. & Mays, J. Macromolecular architectures by living and controlled/living polymerizations. Prog. Polym. Sci. 31, 1068–1132 (2006).
Ménard, A. D. & Trant, J. F. A review and critique of academic lab safety research. Nat. Chem. 12, 17–25 (2020).
Rathman, T. ‘L.’ & Schwindeman, J. A. Preparation, properties, and safe handling of commercial organolithiums: alkyllithiums, lithium sec-organoamides, and lithium alkoxides. Org. Process Res. Dev. 18, 1192–1210 (2014).
Slavík, P., Trowse, B. R., O’Brien, P. & Smith, D. K. Organogel delivery vehicles for the stabilization of organolithium reagents. Nat. Chem. 15, 319–325 (2023).
Ratkanthwar, K., Hadjichristidis, N. & Mays, J. W. in Anionic Polymerization: Principles, Practice, Strength, Consequences and Applications (eds Hadjichristidis, N. & Hirao, A.) 19–60 (Springer, 2015).
Baskaran, D. & Müller, A. H. E. Anionic vinyl polymerization—50 years after Michael Szwarc. Prog. Polym. Sci. 32, 173–219 (2007).
Grubbs, R. B. & Grubbs, R. H. 50th anniversary perspective: living polymerization—emphasizing the molecule in macromolecules. Macromolecules 50, 6979–6997 (2017).
Baskaran, D. Strategic developments in living anionic polymerization of alkyl (meth)acrylates. Prog. Polym. Sci. 28, 521–581 (2003).
Varshney, S. K., Hautekeer, J. P., Fayt, R., Jerome, R. & Teyssie, P. Anionic polymerization of (meth)acrylic monomers. 4. Effect of lithium salts as ligands on the “living” polymerization of methyl methacrylate using monofunctional initiators. Macromolecules 23, 2618–2622 (1990).
Zune, C., Archambeau, C., Dubois, P. & Jérôme, R. Effect of the solvent polarity on the living ligated anionic polymerization of tert-butyl methacrylate and copolymerization with methyl methacrylate. J. Polym. Sci. A 39, 1774–1785 (2001).
Vlček, P. & Lochmann, L. Anionic polymerization of (meth)acrylate esters in the presence of stabilizers of active centres. Prog. Polym. Sci. 24, 793–873 (1999).
Lochmann, L. & Lím, D. Preparation and properties of pure lithio esters of some carboxylic acids. J. Organomet. Chem. 50, 9–16 (1973).
Carlotti, S., Desbois, P., Warzelhan, V. & Deffieux, A. Retarded anionic polymerization (RAP) of styrene and dienes. Polymer 50, 3057–3067 (2009).
Li, Z. et al. Anionic living polymerization of alkyl methacrylate at ambient temperature and its mechanism research. J. Polym. Sci. A 57, 1130–1139 (2019).
Kralisch, D., Ott, D. & Gericke, D. Rules and benefits of life cycle assessment in green chemical process and synthesis design: a tutorial review. Green Chem. 17, 123–145 (2015).
Webster, O. W. in New Synthetic Methods Vol. 167 (eds Abe, A. et al.) 1–34 (Springer, 2003).
McGraw, M. L. & Chen, E. Y.-X. Lewis pair polymerization: perspective on a ten-year journey. Macromolecules 53, 6102–6122 (2020).
Zhang, Y., Miyake, G. M. & Chen, E. Y.-X. Alane-based classical and frustrated Lewis pairs in polymer synthesis: rapid polymerization of MMA and naturally renewable methylene butyrolactones into high-molecular-weight polymers. Angew. Chem. Int. Ed. 49, 10158–10162 (2010).
Zhang, Y. et al. Lewis pair polymerization by classical and frustrated Lewis pairs: acid, base and monomer scope and polymerization mechanism. Dalton Trans. 41, 9119–9134 (2012).
Sanford, M. J., Van Zee, N. J. & Coates, G. W. Reversible-deactivation anionic alternating ring-opening copolymerization of epoxides and cyclic anhydrides: access to orthogonally functionalizable multiblock aliphatic polyesters. Chem. Sci. 9, 134–142 (2018).
Varghese, J. K. et al. A new role for CO2: controlling agent of the anionic ring-opening polymerization of cyclic esters. Macromolecules 50, 6752–6761 (2017).
Abel, B. A., Snyder, R. L. & Coates, G. W. Chemically recyclable thermoplastics from reversible-deactivation polymerization of cyclic acetals. Science 373, 783–789 (2021).
Corrigan, N. et al. Reversible-deactivation radical polymerization (controlled/living radical polymerization): from discovery to materials design and applications. Prog. Polym. Sci. 111, 101311 (2020).
Ouchi, M. & Sawamoto, M. Sequence-controlled polymers via reversible-deactivation radical polymerization. Polym. J. 50, 83–94 (2018).
Uchiyama, M., Ohira, N., Yamashita, K., Sagawa, K. & Kamigaito, M. Proton transfer anionic polymerization with C-H bond as the dormant species. Nat. Chem. 16, 1630–1637 (2024).
Sakakura, T., Choi, J.-C. & Yasuda, H. Transformation of carbon dioxide. Chem. Rev. 107, 2365–2387 (2007).
Kong, D., Moon, P. J., Lui, E. K. J., Bsharat, O. & Lundgren, R. J. Direct reversible decarboxylation from stable organic acids in dimethylformamide solution. Science 369, 557–561 (2020).
Naumann, S., Schmidt, F. G., Schowner, R., Frey, W. & Buchmeiser, M. R. Polymerization of methyl methacrylate by latent pre-catalysts based on CO2-protected N-heterocyclic carbenes. Polym. Chem. 4, 2731–2740 (2013).
Wadhwa, K. et al. Influence of substitution of various functional groups on inhibition efficiency of TEMPO analogues on styrene polymerization. J. Polym. Res. 24, 201 (2017).
Hunter, D. H., Hamity, M., Patel, V. & Perry, R. A. Crown ether catalysis of decarboxylation: a general survey of reactivity and detailed analysis of the triphenylacetate anion. Can. J. Chem. 56, 104–113 (1978).
Kottisch, V., Gentekos, D. T. & Fors, B. P. “Shaping” the future of molecular weight distributions in anionic polymerization. ACS Macro Lett. 5, 796–800 (2016).
Liu, X., Jiang, C., Ren, C. & Li, Z. Controlled ring-opening polymerization of epoxides catalyzed by metal-free phosphazenium salts (P5+): using carboxylic acid as an initiator to prepare esterified polyethers. ACS Appl. Polym. Mater. 6, 896–904 (2023).
Sivakumar, K. et al. A fluorogenic 1,3-dipolar cycloaddition reaction of 3-azidocoumarins and acetylenes. Org. Lett. 6, 4603–4606 (2004).
Binder, W. H. & Sachsenhofer, R. ‘Click’ chemistry in polymer and materials science. Macromol. Rapid Commun. 28, 15–54 (2007).
Kaur, J., Saxena, M. & Rishi, N. An overview of recent advances in biomedical applications of click chemistry. Bioconjugate Chem. 32, 1455–1471 (2021).
Anderson, C. D., Shea, K. J. & Rychnovsky, S. D. Strategies for the generation of molecularly imprinted polymeric nitroxide catalysts. Org. Lett. 7, 4879–4882 (2005).
Bugnon, L., Morton, C. J. H., Novak, P., Vetter, J. & Nesvadba, P. Synthesis of poly(4-methacryloyloxy-TEMPO) via group-transfer polymerization and its evaluation in organic radical battery. Chem. Mater. 19, 2910–2914 (2007).
Amorati, R., Pedulli, G. F., Pratt, D. A. & Valgimigli, L. TEMPO reacts with oxygen-centered radicals under acidic conditions. Chem. Commun. 46, 5139–5141 (2010).
Downie, I. M., Earle, M. J., Heaney, H. & Shuhaibar, K. F. Vilsmeier formylation and glyoxylation reactions of nucleophilic aromatic compounds using pyrophosphoryl chloride. Tetrahedron 49, 4015–4034 (1993).
Armesto, D. et al. Novel photoreactions of 2-aza-1,4-dienes in the triplet excited state and via radical-cation intermediates. 2-aza-di-π-methane rearrangements yielding cyclopropylimines and N-vinylaziridines. J. Org. Chem. 68, 6661–6671 (2003).
Xing, W.-L., Wang, J.-X., Fu, M.-C. & Fu, Y. Efficient decarboxylative/defluorinative alkylation for the synthesis of gem-difluoroalkenes through an SN2’-type route. Chin. J. Chem. 40, 323–328 (2022).
Devasthale, P. et al. Cyclobutane- and azetidine-containing mono and spirocyclic compounds as alpha V integrin inhibitors. International patent WO2018089355A1 (2018).
Cho, D. et al. Temperature gradient interaction chromatography and MALDI-TOF mass spectrometry analysis of stereoregular poly(ethyl methacrylate)s. Anal. Chem. 74, 1928–1931 (2002).
Jacky, P., Easley, A. & Fors, B. Data from: Controlled anionic polymerization mediated by carbon dioxide. Cornell University eCommons Repository https://doi.org/10.7298/s733-8m37 (2025).
Acknowledgements
We thank G. Coates for use of the GPC system with an ultraviolet detector and J. Koehl for sample preparation and analysis. We thank E. Stache for use of the GPC system with N,N-dimethylacetamide as the eluent and C. Preston for sample preparation and analysis. This work was supported by the National Science Foundation (grant number CHE 2203758 to B.P.F., P.E.J. and A.D.E.), National Science Foundation Graduate Research Fellowship Program (grant number DGE 2139899 to P.E.J.) and Cornell College of Arts and Sciences Klarman Postdoctoral Fellowship (to A.D.E.). This work made use of the Cornell University NMR Facility, which is supported in part by the National Science Foundation through MRI award CHE-1531632. This work made use of the Cornell Center for Materials Research, supported by the Materials Research Science and Engineering Centers programme DMR-1120296. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
P.E.J., A.D.E. and B.P.F. designed the experiments and prepared the paper. P.E.J. and A.D.E. conducted and analysed the experiments.
Corresponding author
Ethics declarations
Competing interests
On 3 March 2025, B.P.F. and P.E.J. filed a patent application (US Patent application number 63/765,983; ‘Vinyl polymers, methods of making same, and uses thereof’) on technology related to the processes described in this article. The other author declares no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Jeffrey Foster and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Methods, Figs. 1–78, Tables 1–10 and References.
Source data
Source Data Fig. 3
Numerical data for the plots in Fig. 3b–d.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jacky, P.E., Easley, A.D. & Fors, B.P. Controlled anionic polymerization mediated by carbon dioxide. Nat. Chem. 17, 1076–1082 (2025). https://doi.org/10.1038/s41557-025-01819-7
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01819-7