Abstract
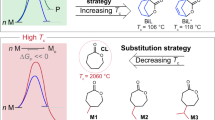
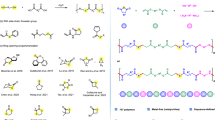
Monomer design strategy has become a powerful tool to access chemically recyclable polymers with desired and diverse properties. The presence of two or multiple stereogenic centres in one monomer offers a new dimension to fine-tune the polymer performance. However, it is still a formidable challenge in synthetic polymer chemistry to achieve precise stereocontrol and sequence control over the polymer microstructure. Here we report a stereo- and sequence-controlled polymerization of 5H-1,4-benzodioxepin-3(2H)-one-based monomers with two stereogenic centres (M) to furnish a series of isoenriched AB diblock polymers P(cis-M)-b-P(trans-M) and ABA triblock polymers P(trans-M)-b-P(cis-M)-b-P(trans-M). Notably, P(cis-M2)900-b-P(trans-M2)38 delivered impressive toughness and ductility, comparable to the commodity plastic isotactic polypropylene; the ABA triblock P(trans-M2)26-b-P(cis-M2)900-b-P(trans-M2)26 appeared to be softer and resembled low-density polyethylene. These various materials could fully convert to the monomer M. The establishment of stereo- and sequence-controlled polymerization not only provides an effective and robust strategy to tailor polymer properties on the molecular level, but also delivers various chemically recyclable materials that can be converted back to monomers.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that the data supporting the findings of this study are provided in the main Article and the Supplementary Information and are also available from the authors upon reasonable request. Crystallographic data for the structure of (R,R)-M1 reported in this Article have been deposited at the Cambridge Crystallographic Data Centre under deposition number CCDC 2367052. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Geyer, R., Jambeck, J. R. & Law, K. L. Production, use, and fate of all plastics ever made. Sci. Adv. 3, 25–29 (2017).
MacLeod, M., Arp, H. P. H., Tekman, M. B. & Jahnke, A. The global threat from plastic pollution. Science 373, 61–65 (2021).
Rosenboom, J.-G., Langer, R. & Traverso, G. Bioplastics for a circular economy. Nat. Rev. Mater. 7, 117–137 (2022).
Hong, M. & Chen, E. Y.-X. Chemically recyclable polymers: a circular economy approach to sustainability. Green Chem. 19, 3692–3706 (2017).
Xu, G. & Wang, Q. Chemically recyclable polymer materials: polymerization and depolymerization cycles. Green Chem. 24, 2321–2346 (2022).
Law, K. L. & Narayan, R. Reducing environmental plastic pollution by designing polymer materials for managed end-of-life. Nat. Rev. Mater. 7, 104–116 (2022).
Haque, F. M. et al. Defining the macromolecules of tomorrow through synergistic sustainable polymer research. Chem. Rev. 122, 6322–6373 (2022).
Coates, G. W. & Getzler, Y. D. Y. L. Chemical recycling to monomer for an ideal, circular polymer economy. Nat. Rev. Mater. 5, 501–516 (2020).
Shi, C., Quinn, E. C., Diment, W. T. & Chen, E. Y.-X. Recyclable and (bio)degradable polyesters in a circular plastics economy. Chem. Rev. 124, 4393–4478 (2024).
Shi, C. et al. Design principles for intrinsically circular polymers with tunable properties. Chem 7, 2896–2912 (2021).
Li, X.-L., Ma, K., Xu, F. & Xu, T.-Q. Advances in the synthesis of chemically recyclable polymers. Chem. Asian J. 18, e202201167 (2023).
Zhou, L., Reilly, L. T., Shi, C., Quinn, E. C. & Chen, E. Y.-X. Proton-triggered topological transformation in superbase-mediated selective polymerization enables access to ultrahigh-molar-mass cyclic polymers. Nat. Chem. 16, 1357–1365 (2024).
Hughes, R. W. et al. Bulk depolymerization of methacrylate polymers via pendent group activation. J. Am. Chem. Soc. 146, 6217–6224 (2024).
Chin, M. T. et al. Implementing a doping approach for poly(methyl methacrylate) recycling in a circular economy. J. Am. Chem. Soc. 146, 5786–5792 (2024).
Wang, Y., Zhu, Y., Lv, W., Wang, X. & Tao, Y. Tough while recyclable plastics enabled by monothiodilactone monomers. J. Am. Chem. Soc. 145, 1877–1885 (2023).
Zhou, L. et al. Chemically circular, mechanically tough, and melt-processable polyhydroxyalkanoates. Science 380, 64–69 (2023).
Rapagnani, R. M., Dunscomb, R. J., Fresh, A. A. & Tonks, I. A. Tunable and recyclable polyesters from CO2 and butadiene. Nat. Chem. 14, 877–883 (2022).
Abel, B. A., Snyder, R. L. & Coates, G. W. Chemically recyclable thermoplastics from reversible-deactivation polymerization of cyclic acetals. Science 373, 783–789 (2021).
Li, Z., Shen, Y. & Li, Z. Ring-opening polymerization of lactones to prepare closed-loop recyclable polyesters. Macromolecules 57, 1919–1940 (2024).
Kariyawasam, L. S., Rolsma, J. & Yang, Y. Chemically recyclable dithioacetal polymers via reversible entropy-driven ring-opening polymerization. Angew. Chem. Int. Ed. 62, e202303039 (2023).
Meng, X.-B. et al. Thermally stable and chemically recyclable poly(ketal-ester)s regulated by floor temperature. J. Am. Chem. Soc. 146, 15428–15437 (2024).
Hu, Z. et al. Catalytically controlled ring-opening polymerization of 2-oxo-15-crown-5 for degradable and recyclable PEG-like polyesters. ACS Macro Lett. 11, 792–798 (2022).
Tu, Y.-M., Gong, F.-L., Wu, Y.-C., Cai, Z. & Zhu, J.-B. Insights into substitution strategy towards thermodynamic and property regulation of chemically recyclable polymers. Nat. Commun. 14, 3198 (2023).
Li, X.-L., Clarke, R. W., Jiang, J.-Y., Xu, T.-Q. & Chen, E. Y.-X. A circular polyester platform based on simple gem-disubstituted valerolactones. Nat. Chem. 15, 278–285 (2023).
Xiong, W. & Lu, H. Recyclable polythioesters and polydisulfides with near-equilibrium thermodynamics and dynamic covalent bonds. Sci. China Chem. 66, 725–738 (2023).
Sathe, D., Yoon, S., Wang, Z., Chen, H. & Wang, J. Deconstruction of polymers through olefin metathesis. Chem. Rev. 124, 7007–7044 (2024).
Shi, C., Diment, W. & Chen, E. Y.-X. Closed-loop recycling of mixed plastics of polyester and CO2-based polycarbonate to a single monomer. Angew. Chem. Int. Ed. 63, e202405083 (2024).
Thomas, C. M. Stereocontrolled ring-opening polymerization of cyclic esters: synthesis of new polyester microstructures. Chem. Soc. Rev. 39, 165–173 (2010).
Feng, L., Bian, X., Li, G. & Chen, X. Thermal properties and structural evolution of poly(l-lactide)/poly(d-lactide) blends. Macromolecules 54, 10163–10176 (2021).
Li, H., Ollivier, J., Guillaume, S. M. & Carpentier, J.-F. Tacticity control of cyclic poly(3-thiobutyrate) prepared by ring-opening polymerization of racemic β-thiobutyrolactone. Angew. Chem. Int. Ed. 61, e202202386 (2022).
Tang, X. & Chen, E. Y.-X. Chemical synthesis of perfectly isotactic and high melting bacterial poly(3-hydroxybutyrate) from bio-sourced racemic cyclic diolide. Nat. Commun. 9, 2345 (2018).
He, C. et al. Monomer-promoting asymmetric kinetic resolution-alternating copolymerization to afford AAB-type copolyesters. J. Am. Chem. Soc. 145, 9786–9799 (2023).
Li, K. et al. Kinetic resolution polymerization enabled chemical synthesis of perfectly isotactic polythioesters. Angew. Chem. Int. Ed. 63, e202405382 (2024).
Guo, X., Xu, G., Yang, R. & Wang, Q. Specific discrimination polymerization for highly isotactic polyesters synthesis. J. Am. Chem. Soc. 146, 9084–9095 (2024).
Lutz, J.-F., Ouchi, M., Liu, D. R. & Sawamoto, M. Sequence-controlled polymers. Science 341, 1238149 (2013).
Rosen, T., Goldberg, I., Navarra, W., Venditto, V. & Kol, M. Block–stereoblock copolymers of poly(ϵ-caprolactone) and poly(lactic acid). Angew. Chem. Int. Ed. 57, 7191–7195 (2018).
Wang, X., Chin, A. L., Zhou, J., Wang, H. & Tong, R. Resilient poly(α-hydroxy acids) with improved strength and ductility via scalable stereosequence-controlled polymerization. J. Am. Chem. Soc. 143, 16813–16823 (2021).
Liu, Y. & Lu, X.-B. Current challenges and perspectives in CO2-based polymers. Macromolecules 56, 1759–1777 (2023).
Tschan, M. J.-L., Gauvin, R. M. & Thomas, C. M. Controlling polymer stereochemistry in ring-opening polymerization: a decade of advances shaping the future of biodegradable polyesters. Chem. Soc. Rev. 50, 13587–13608 (2021).
Liu, X., Hua, X. & Cui, D. Copolymerization of lactide and cyclic carbonate via highly stereoselective catalysts to modulate copolymer sequences. Macromolecules 51, 930–937 (2018).
Li, H., Shakaroun, R. M., Guillaume, S. M. & Carpentier, J. F. Recent advances in metal-mediated stereoselective ring-opening polymerization of functional cyclic esters towards well-defined poly(hydroxy acid)s: from stereoselectivity to sequence-control. Chem. Eur. J. 26, 128–138 (2020).
Liao, X., Su, Y. & Tang, X. Stereoselective synthesis of biodegradable polymers by salen-type metal catalysts. Sci. China Chem. 65, 2096–2121 (2022).
Huang, H.-Y. et al. Spiro-salen catalysts enable the chemical synthesis of stereoregular polyhydroxyalkanoates. Nat. Catal. 6, 720–728 (2023).
Deacy, A. C., Gregory, G. L., Sulley, G. S., Chen, T. T. D. & Williams, C. K. Sequence control from mixtures: switchable polymerization catalysis and future materials applications. J. Am. Chem. Soc. 143, 10021–10040 (2021).
Chellali, J. E. et al. Access to stereoblock polyesters via irreversible chain-transfer ring-opening polymerization (ICT-ROP). J. Am. Chem. Soc. 146, 11562–11569 (2024).
Jia, Z., Li, Y. & Wu, J. Sequence-controlled alternating copolyesters synthesis via selective ring-opening polymerization. Macromol. Chem. Phys. 222, 2100323 (2021).
Tang, X., Westlie, A. H., Watson, E. M. & Chen, E. Y.-X. Stereosequenced crystalline polyhydroxyalkanoates from diastereomeric monomer mixtures. Science 366, 754–758 (2019).
Zhou, Z., LaPointe, A. M., Shaffer, T. D. & Coates, G. W. Nature-inspired methylated polyhydroxybutyrates from C1 and C4 feedstocks. Nat. Chem. 15, 856–861 (2023).
Tu, Y.-M. et al. Biobased high-performance aromatic–aliphatic polyesters with complete recyclability. J. Am. Chem. Soc. 143, 20591–20597 (2021).
Tang, Z. et al. Stereoselective polymerization of rac-lactide using a monoethylaluminum Schiff base complex. Biomacromolecules 5, 965–970 (2004).
Wang, X. et al. Bayesian-optimization-assisted discovery of stereoselective aluminum complexes for ring-opening polymerization of racemic lactide. Nat. Commun. 14, 3647 (2023).
McGuire, T. M. et al. Control of crystallinity and stereocomplexation of synthetic carbohydrate polymers from d- and l-xylose. Angew. Chem. Int. Ed. 60, 4524–4528 (2021).
Wan, Z.-Q. et al. Comprehensive understanding of polyester stereocomplexation. J. Am. Chem. Soc. 141, 14780–14787 (2019).
Zhu, J.-B., Watson, E. M., Tang, J. & Chen, E. Y.-X. A synthetic polymer system with repeatable chemical recyclability. Science 360, 398–403 (2018).
Tsuji, H. Poly(lactic acid) stereocomplexes: a decade of progress. Adv. Drug Deliv. Rev. 107, 97–135 (2016).
Xia, Y., Yuan, P., Zhang, Y., Sun, Y. & Hong, M. Converting non-strained γ-valerolactone and derivatives into sustainable polythioesters via isomerization-driven cationic ring-opening polymerization of thionolactone intermediate. Angew. Chem. Int. Ed. 62, e202217812 (2023).
Fan, H.-Z. et al. Advancing the development of recyclable aromatic polyesters by functionalization and stereocomplexation. Angew. Chem. Int. Ed. 61, e202117639 (2022).
Worch, J. C. et al. Stereochemical enhancement of polymer properties. Nat. Rev. Chem. 3, 514–535 (2019).
Cheng, M., Attygalle, A. B., Lobkovsky, E. B. & Coates, G. W. Single-site catalysts for ring-opening polymerization: synthesis of heterotactic poly(lactic acid) from rac-lactide. J. Am. Chem. Soc. 121, 11583–11584 (1999).
Acknowledgements
This work was supported by the National Key R&D Program of China (2021YFA1501700), the National Natural Science Foundation of China (22071163, 22301197 and 22371194), and the Fundamental Research Funds from Sichuan University (2023SCUNL103) and the State Key Laboratory of Polymer Materials Engineering (sklpme2020-3-15). We thank P.-C. Deng, D. Deng and J. Li for compound testing.
Author information
Authors and Affiliations
Contributions
J.-B.Z. conceived the project. Z.C. and J.-B.Z. directed the research. M.-Y.W. designed and conducted experiments related to polymer synthesis, polymer characterizations and catalyst synthesis. Y.-M.T. and Q.-Q.Z. conducted experiments related to polymer synthesis and characterizations. K.L. and W.X. conducted catalyst synthesis. M.-Y.W., Z.C. and J.-B.Z. wrote the initial paper and all authors contributed to the data analysis and discussions and the revised paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Claudio De Rosa and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–186, discussion and Tables 1–31.
Supplementary Data 1
Crystallographic data for (R,R)-M1 (CCDC 2367052).
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, MY., Tu, YM., Zeng, QQ. et al. High-performance recyclable polymers enabled by stereo- and sequence-controlled polymerization. Nat. Chem. 17, 1119–1128 (2025). https://doi.org/10.1038/s41557-025-01828-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01828-6