Abstract
Post-translational modifications such as phosphorylation and acetylation are often minor structural modifications that can have profound effects on protein structure and thus broaden protein functions. Nevertheless, studying these effects directly is often out of reach because no general chemistry exists to introduce small modifications selectively; either a large, stable linker structure is selectively installed on protein residues, or a small substituent is introduced at the risk of low selectivity due to the use of reactive, indiscriminate molecules. Here we report a C–H functionalization reaction of tyrosine residues to access peptides and proteins modified by small structural changes including single-atom substitutions. A rationally designed selenoxide introduces a versatile selenonium linchpin featuring a Ctyr–Se bond that can be used for further transformations at specific tyrosine residues. Key to the advance is the interplay of water-resistant, intramolecular chalcogen and hydrogen bonding of the selenoxide reagent, which allows chemo- and site-selective electrophilic aromatic substitution of tyrosine residues in aqueous solutions.

Similar content being viewed by others
Main
Post-translational modifications (PTMs) of proteins and peptides play multifaceted roles in numerous biochemical processes, including signal transduction1,2, metabolic regulation3 and cell proliferation3,4. Chemically modified peptides and proteins are also valuable in imaging, biomaterials and drug delivery5,6,7, and even single-atom modifications can have a profound impact such as increased cellular uptake8 and enhanced receptor binding9. Peptides and proteins with PTMs are mainly obtained by expression from specific cell lines or by in vitro enzymatic modification10. However, the range of peptidyl substrates and the types of modification are restricted by the genetic machinery and enzyme functions11. Chemical synthesis and semisynthesis of proteins with PTMs by native chemical ligation12 and by expressed protein ligation9,13 are complementary approaches, but are work intensive13. Chemical methods for the introduction of small structural perturbations to native proteins are attractive because they could expand the scope of modified products while also avoiding complex molecular biology operations and laborious protein synthesis. Due to the stringent requirements of large biomolecules, including the necessity for an aqueous environment within a small temperature window, a low (micromolar) concentration of the protein substrate, and site- and chemoselectivity11,14,15, chemical methods that can selectively functionalize endogenous proteins or peptides with only small structural perturbations, for example, without a linker structure, are scarce. The Davis group reported versatile post-translational protein editing by chemoselective functionalization of cysteine residues that, with the appropriate chemistry, results in synthetically useful dehydroalanine substituents16 or radicals17 for further diversification. Modification of native proteins can also be accomplished with small, reactive molecules, such as light-induced nitration18 or halogenation19. Such chemistry can be chemoselective but the energetic small molecules used in this chemistry such as nitrogen dioxide and halogen radicals can make the control of site-selectivity difficult. Chemo- and site-selectivity are already challenging for small-molecule C–H functionalization, and the challenges are exacerbated for late-stage functionalization of polypeptides and proteins. Despite the utility of current chemical20,21,22 and biochemical11,14,15 late-stage functionalization, the regioselective introduction of a linchpin by C–H functionalization chemistry for subsequent diversification, including single-atom modifications, is currently unknown in the field of bioconjugation, yet would afford valuable probes and analogues with small structural changes.
Tyrosine residues are an attractive target for site-selective modification due to their small surface abundance23. Small modifications of tyrosine residues enable fine-tuning of the pKa of the phenolic hydroxyl group24, the nucleophilicity of phenolate25 and the redox potential of the tyrosine π system24,26, which can impact the activity of peptidyl drugs9,27 and protein–protein interactions8. However, current chemical methods are generally not suitable for functionalization of peptides and proteins with small and single-atom modifications on tyrosine, in part because the desired atoms cannot be selectively introduced directly18,19 and because the larger groups that can be introduced selectively form stable C–C (ref. 28), C–O (ref. 29) or C–N (ref. 30) bonds that cannot be readily cleaved subsequently. In general, the use of mild reagents due to the restrictions of selectivity and conditions leads to the formation of a stable linkage on the modified residues. Based on our knowledge, the chemoselective introduction of convertible substituents with a weak, cleavable linkage is only achieved on the side chains of cysteine16,17, selenocysteine31,32 or selenomethionine33 residues. In other words, heavy main-group elements such as sulfur and selenium must be involved in such linkages.
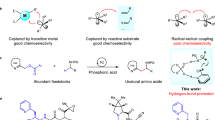
We had previously developed C–H thianthrenation chemistry for small molecules that is distinguished by exquisite site- and regioselectivity and by the large functional group tolerance34. Combined with the ability to convert the thianthrenium linchpin into a multitude of substituents34,35,36,37, such an approach would be ideal for small and single-atom peptide and protein modification. However, the reactive intermediate in arene thianthrenation is the aromatic, thianthrene dication, which does not persist sufficiently long in water for productive C–H functionalization in an aqueous environment38,39. Functionalization can be induced by acylation of thianthrene S-oxide (TT-OTFA+) or strong acid in non-aqueous medium but neither can be used for most complex biomolecules (Fig. 1a). With an analogue more basic than thianthrene S-oxide for protonation in water, one might be able to benefit from the high chemo- and stereoselectivity of thianthrenation which can distinguish subtle differences in the electronic structure of electron-rich arenes as indicated by a Hammett ρ value of −11 (ref. 39). However, no sulfoxide that we evaluated reached sufficiently high basicity to achieve productive C–H functionalization for potential protein modification in water (Supplementary Table 1). Therefore, we evaluated sulfur’s heavier homologue selenium due to its higher basicity40. What resulted ultimately is a selenoxide-based reagent 1 whose protonated form 1H+ can react productively in water, yet still retain high positional selectivity, so that selective C–H functionalization of tyrosine residues of peptidyl substrates becomes feasible (Fig. 1b). The selenium-based substituent in the obtained tyrosine-based arylselenonium salts can be converted into a variety of other substituents, including single atoms to access simple, well-defined analogues that are challenging to access otherwise. Known selenium chemistry in protein science41,42 uses the nucleophilicity of selenols31,32 and selenides33, and selenoxides are used for disulfide bond formation43; this work shows the application of an electrophilic selenium reagent to C–H functionalization of biomolecules. The weak C(sp2)–Se bond can readily be engaged for further transformations and provides insights into the utilization of heavy main-group elements in the field of bioconjugation.
a, Thianthrenation: although versatile and highly selective, functionalization with the water-sensitive TT-OTFA+ can only proceed under anhydrous conditions. b, This work: positional-selective, versatile single-atom protein modification with a selenium-based electrophile. The more basic selenoxide group, and the combination of the water-resistant, intramolecular chalcogen and hydrogen bonding, leads to the water-stable electrophile 1H+, thus enabling modification in water. Ac, acetyl; TT, thianthrene; TFA, trifluoroacetyl group; TM, transition metal; X, functional group (for example, halogenyl, hydroxyl).
Results and discussion
Design and mechanistic study of selenoxide 1
A productive functionalization of tyrosine analogue 2 with thianthrene S-oxide 3 could not be observed even at pH 1.0 in water due to the insufficient basicity of 3 (Fig. 2a). As a proof of principle, the simple selenoxide 4, which is more basic than the sulfoxides, could functionalize 2, although only at an inappropriately low pH of 1 that was necessary to access the selenoxonium 4H+, which we anticipate is the reactive electrophile for aromatic functionalization (Fig. 2b and Supplementary Figs. 15 and 16). We sought to design structural modifications of 4 that would increase the pKa value of the conjugate acid sufficiently that productive C–H functionalization could proceed efficiently in sodium citrate buffer at pH 3. A proximal Lewis base could act as a chalcogen bond acceptor to increase electron density at selenium44 and thereby the basicity of the selenoxide for protonation at higher pH. In addition, the chalcogen bond should induce a low-energy conformation45 that we suspected may preorganize the selenoxonium hydroxyl group for potential hydrogen bonding to an intramolecular hydrogen-bond acceptor. The hydrogen bond would further stabilize the selenoxonium cation to elicit electrophilic C–H functionalization at higher pH. Introduction of an appropriately positioned pyridyl substituent in 5 resulted in a higher pKa value and is consistent with a Se–N chalcogen bond in 5H+. NMR studies are consistent with the existence of the chalcogen bond in aqueous solution; the 77Se NMR resonances of 5 and 5H+ are shifted by about 6 ppm when compared to 4 and 4H+, respectively, which has also been observed for other compounds with chalcogen bonds46 (Fig. 2c). Additionally, the intramolecular chalcogen bond in 5H+ could be observed in the solid state, where a short Se–N distance of 2.62 Å was observed; the sum of the van der Waals radii is 3.45 Å (ref. 47). The chalcogen bond, rather than the electron-withdrawing property of the pyridyl group, is critical for the improved reactivity of 5 at pH 3.0, as indicated by the inertness of an analogue in which the DBSe scaffold is para to the pyridyl nitrogen towards 2 under the same conditions (Supplementary Table 1, entry 6). Introduction of an additional substituent appropriately situated to engage in hydrogen bonding with a protonated selenoxide resulted in amide 6. NMR studies indicate that the amide group of 6H+ is hydrogen bonded to a donor more acidic than water, which is possibly the intramolecular selenoxonium group (Fig. 2d); the larger 15N NMR resonance shift of the amide group of 6H+ compared to 6 (Δδ = 2.2 ppm; Fig. 2d) matches the reported shift values of other amides with hydrogen-bond donor shift from water to more acidic trifluoroethanol (Δδ = 0.8–2.9 ppm)48. Additionally, the shifts of 13C NMR and 1H NMR resonances of the amide group of 6H+ compared to 6 are consistent with the existence of an intramolecular hydrogen bond (Supplementary Figs. 22 and 24–26). Nevertheless, the introduction of the hydrogen bond to 6H+ only slightly increased the desired reactivity at about the same acidity, with a pKa of 6H+ similar to that of 5H+. Because of the weak basicity of the amide group, the additional stabilization of 6H+ by the hydrogen bond may be modest and further offset by a weaker intramolecular chalcogen bond due to the lower Lewis basicity of the amide-substituted pyridyl group. To combine both advantageous effects, that is, chalcogen and hydrogen bonding, we designed selenoxide 1 with an oxazolyl group instead of the amide in 6. The oxazolyl group is more basic than the amide group in 6, which resulted in improved stabilization of 1H+ and thereby the higher pKa and productive C–H functionalization in 88% yield at pH 3. Replacement of the oxazolyl group with an even more basic pyridyl group was inefficient for C–H functionalization at pH 3 (Supplementary Table 1, entry 11), perhaps due to protonation of the distant pyridyl group rather than the selenoxide. The crystal structure of 1H+HSO4–·(MeOH)2 with a refined hydrogen atom confirmed the presence of the intramolecular hydrogen bond between the oxazolyl group and the selenoxonium group in the solid state, in addition to the intramolecular chalcogen bond. The smaller 15N NMR resonance of the oxazolyl group of 1H+ compared to 1 (Δδ = −10.5 ppm; Fig. 2e) in aqueous solution indicates the increased shielding at the nitrogen nucleus of 1H+, explained by the change of the contribution of the sp2-hybridized nitrogen lone pair to the overall shielding tensor, which is induced by the protonation of or the hydrogen bonding to the nitrogen atom of the oxazolyl group49. We attribute the observed reactivity to the combination of both chalcogen and hydrogen bonds; simple activation through a second protonation event of 1H+ at the oxazolyl group at pH 1, in which the protonated oxazolyl group would merely function as an intramolecular Brønsted acid, is not supported by the titration experiment shown in Fig. 2f, which indicates a single protonation event and is consistent with a hydrogen bond that is stable even in water. Similarly, a control experiment with the structural components of 1 but intermolecular, namely an equimolar mixture of oxazole and 5, which lack the hydrogen-bonding interaction between them as judged by the nearly identical 77Se NMR resonances in the NMR study of selenoxide 5 (Fig. 2c) and in the control experiment (Fig. 2g), is consistent with the intramolecular hydrogen bond in 1H+. The 15N NMR shift of the oxazole at pH 1 in the presence of 5H+ decreases only by 3 ppm compared to neutral oxazole (Fig. 2g). The comparison to the change Δδ = −10.5 ppm for 1 (Fig. 2e) suggests that hydrogen bonding between oxazole and hydrated protons is insufficient to explain the observed 15N shifts in 1H+ when compared to 1. We therefore conclude that a water-resistant intramolecular hydrogen bond between the oxazolyl nitrogen and the protonated selenoxonium group can rationalize the higher reactivity of 1H+ in electrophilic substitution in tyrosine residues.
a, Reaction yield as a function of pKa and pH. Aqueous H2SO4 used for pH 1.0; sodium citrate buffer used for pH 3.0. Yield determined by 1H NMR spectroscopy in triplicate. aMeCN 20% (v/v) as cosolvent. b, NMR studies of 4. c, NMR studies of 5 and X-ray structure of 5H+MsO‒. d, NMR studies of 6. e, NMR studies of 1 and X-ray structure of 1H+HSO4‒·(MeOH)2. f, Titration curve of 1. Absorption (A′) measured at 348 nm and corrected for the concentration of 1 during titration (Supplementary Fig. 12). g, Control NMR experiment of a mixture of 5 and oxazole in a molar ratio of 1/1. NMR spectroscopy performed in water/MeCN-d3 of either pH 1 or 13; hydrogen atoms in X-ray structures were found and refined. Ac, acetyl; MeCN, acetonitrile; MsO‒, methanesulfonate anion; MeOH, methanol; Nox, nitrogen nucleus of oxazolyl groups or oxazoles; Npy, nitrogen nucleus of pyridyl groups.
Chemo- and site-selective bioconjugation
The combination of hydrogen and chalcogen bonds in the electrophilic, protonated form of 1 enabled chemoselective C–H functionalization of peptides under aqueous conditions (Fig. 3). For example, the small, cyclic nonapeptide hormone oxytocin featuring a disulfide bond was modified (7, 92%) at pH 3 and 30 °C. Likewise, the anticoagulent drug bivalirudin afforded 8 in 92% yield under the standard conditions. Unlike many selenoxides, which form 4-coordinated selenuranes with carboxylate groups under acidic conditions, selenoxide 1 does not engage in this undesired side reaction with both side-chain and C-terminal carboxylates, perhaps because the dibenzoselenophene scaffold creates a structurally fixed C–Se–C bond angle of 87° (Supplementary Information, page 167), which is smaller than what would be expected in selenuranes (101°)50. Angiotensin I and the 37-mer peptidyl drug pramlintide were modified under the standard conditions in 77% and 57% yield, respectively. The compatibility of other residues not covered by the peptidyl substrates (tryptophans, methionines and cysteines) was also studied by utilizing the corresponding amino acids as proxy at pH 3 and 30 °C (Supplementary Information, pages 80–83). Methionines were tolerated (Supplementary Fig. 30) and the electron-rich tryptophans remained untouched, but a slow conversion of 1 to the corresponding selenide was observed in 46% yield, albeit at a rate that did not preclude tyrosine functionalization. Cysteines were quantitatively oxidized to cystines, even at a temperature of 25 °C and within only 30 min. Given the low surface abundance of free cysteines in proteins (<1.9%)23, we do not envisage that such side reactivity would generally preclude the practical application of this modification method. Based on the favourable reaction on peptides, we examined the C–H functionalization of the 5.8-kDa peptide hormone insulin, which features four tyrosine residues. All tyrosine residues are located on the protein surface, but in different microenvironments, which results in differences large enough to elicit a site-specific functionalization: residues Y26 on the B chain and Y14 on the A chain are less structurally shielded than Y16 on the B chain and Y19 on the A chain in acidic aqueous solutions (Supplementary Information, pages 105–106) and could be functionalized selectively (Supplementary Figs. 43 and 44 and Supplementary Tables 25–27). Insulin was modified in 62% yield including mono- and double-Se-modified products in a ratio of 2.6:1. Protein recovery was 85% with unmodified insulin as the remaining mass balance (Supplementary Fig. 42c). The 8.5-kDa protein ubiquitin, with a tyrosine residue (Y59) that is not surface-exposed but half-buried by surrounding charged residues (Supplementary Fig. 48) and oxidized methionine residues (Supplementary Fig. 36), remains unreactive toward the selenoxide reagent 1 under the standard conditions. Yet, upon denaturation with urea (2.4 M) at 37 °C, the Y59 was modified in 56% yield with a protein recovery of 84% and the remaining mass balance being unmodified ubiquitin (Supplementary Fig. 47). We could further show the selectivity of the functionalization by studying the transformation of larger proteins with multiple tyrosine residues. For example, ribonuclease A (13.7 kDa, 6Y) afforded 13 in 60% yield with a selectivity for mono- versus double-Se-modified products of 11:1 and Y73 and Y115 as the major modification sites (Supplementary Table 29); four methionine residues remained untouched. Human lysozyme (14.4 kDa, 6Y), which features five tryptophan residues, can be modified in 64% yield under the standard conditions with Y45 as the major modification site (Supplementary Table 30).
Yield determined by UV absorption (λmax = 328 nm); yields and ratios of 11 and 13 were determined by UV absorption at 328 nm and deconvoluted zero-charge mass spectra analysis (Supplementary Information, pages 101–102, 113). The numbers in brackets indicate the molecular weight of unmodified proteins. aMgCl2 (0.1 M) was used in the reaction. bTFA (100 mM) was used as buffer. The reaction was conducted at 25 °C for 2.5 h to prevent the aggregation of insulin. cMgCl2 (50 mM) and urea (2.4 M) were used for denaturation. The reaction was conducted at 37 °C for 12 h. dThe reaction was conducted at 37 °C for 16 h; Na2SO4 (200 mM) was used to stabilize the protein. eThe reaction was conducted for 16 h. NaPi, sodium-phosphate buffer; TFA, trifluoroacetic acid; Y, tyrosine residue. Blue background, major modified residue as opposed to red background.
Diversification of tyrosine-selenonium linchpin
The lowest unoccupied molecular orbital of the tyrosine-based selenonium salt 15 has a large contribution from the antibonding Ctyr–Se orbital (Supplementary Fig. 74). Due to the relatively small Ctyr–Se bond dissociation energy of only about 71 kcal mol−1 (Supplementary Fig. 73), and the absorption of 15 in the range of 365–465 nm (Supplementary Fig. 70), we anticipated based on our experience with arylthianthrenium salts51 that homolytic cleavage of the Ctyr–Se bond could occur upon irradiation with visible light without the need for a photocatalyst. The resulting synthetically useful aryl radical should be able to participate in various radical reactions, while the selenyl radical cation could be reduced to the selenide by a sacrificial reductant present in solution (Supplementary Fig. 29). Based on this design, we explored the transformations of selenonium salt 15 in buffered aqueous solution (Fig. 4a). All reactions were conducted on 0.32 μmol with a concentration of 15 of 1.6 mM, similar to the required conditions for peptide or protein transformations. Irradiation at 390 nm for only 10 min in the presence of sodium iodide resulted in iodination of 15 with isopropyl iodide to give 16 in 84% yield, determined by 1H NMR spectroscopy, presumably via halogen-atom transfer52. The selenium scaffold was recovered in a mechanistic probe experiment on a larger scale as the corresponding selenide in 90% yield and the formation of iodine was observed (Supplementary Information, page 69), consistent with the proposed formation of selenyl radicals. In the absence of sodium iodide, full conversion of the selenonium salt 15 was observed but no product was formed (Supplementary Table 15, entry 6), which could result from the oxidative decomposition of tyrosine products by the selenyl radical cation. Bromination and chlorination with Cu(MeCN)4BF4 as the reductant and CuBr2 or CuCl2 as the halogen source, respectively, afforded the other arylhalides 17 and 18 as single-atom modifications. Biologically relevant hydroxylation (19) can be achieved via borylation followed by the oxidation of the intermediate boronate with hydrogen peroxide. A light-mediated Meerwein arylation with a malonate Michael acceptor followed by immediate ring closure leads to the formation of a fluorescent coumarin group in 57% yield (20). All photoreactions proceeded within 10–20 min. Apart from photoredox reactions, the selenonium salt 15 is competent for transition-metal-catalysed cross-couplings due to the low-lying Ctyr–Se antibonding orbital, as evidenced by the palladium-catalysed Suzuki cross-coupling, which afforded biaryl tyrosinamide 21 in 75% yield within 5 min at 37 °C. The combination of a variety of different photomediated and transition-metal-catalysed reactions demonstrates the synthetic versatility of the selenium substituent. We also conducted those reactions on a larger scale and the yields of the isolated products generally agree well with the yields measured by 1H NMR spectroscopy for the reactions conducted on a small scale (Supplementary Information, page 68).
a, Transformations of 15. Yields were determined by crude 1H NMR. aNaI, iPrI. bCuBr2, Cu(MeCN)4BF4, KBr. cCuCl2, Cu(MeCN)4BF4, NaCl. dB2(OH)4, NaF, Hantzsch ester, then H2O2. eFeSO4·7H2O, dimethyl 2-(methoxymethylene)malonate, then EDTA-Na2 and NaHCO3. fPd(OAc)2, (4-fluorophenyl)boronic acid. b, Transformations of selenonium bioconjugates. Yields were determined by UV absorption at 214 nm and, if necessary, deconvoluted zero-charge mass spectra. gNaI, iPrI. hCuBr2, Cu(MeCN)4PF6, KBr. iFeSO4·7H2O, dimethyl 2-(methoxymethylene)malonate, then Na-glycine buffer (pH 9.0). jPd(OAc)2, TPPTS, (4-fluorophenyl)boronic acid. kNaI, iPrI. lCuBr2, Cu(MeCN)4PF6, KBr. All reactions were conducted in aqueous buffer containing organic cosolvent 10–40% (v/v). Reactions, except (f) and (j), were irradiated by 390-nm LEDs for 10–20 min. iPr, isopropyl; MeCN, acetonitrile; Ac, acetyl; TPPTS, 3,3′,3′′-phosphanetriyltris(benzenesulfonic acid) trisodium salt.
Extension of the reaction chemistry to the selenium-modified tyrosine residues in complex peptides and proteins demonstrates the bio-orthogonal reactivity of the selenonium linchpin (Fig. 4b). For example, single-atom modification of oxytocin afforded the iodinated analogue 22 in 50% yield. Likewise, bromination, coumarin formation and Suzuki cross-coupling reactions were carried out on bivalirudin–selenonium conjugate 8, and the yields were determined by measuring the absorption at 214 nm using bivalirudin as a reference. Coumarin formation allows the direct post-translational mutation of a tyrosine residue into a fluorescent residue close to the peptide backbone with only a small change of the residue size. Post-translational coumarin introduction may provide the opportunity to study protein solution structure based on probing the distance between different residues via fluorescence resonance energy transfer53. Iodo-insulin 26 was obtained in 64% yield including mono- and double-iodinated products with a ratio of 3.6:1. The dominant modification site is the Y26 residue upon iodination, and an additional iodination site Y16 was observed, which could possibly result from the iodination of small amounts of selenium-modified Y16 originating from the previous selenium-modification step (Supplementary Figs. 42–44). The possibility that the iodinated Y16 results from the side reaction between unmodified Y16 and the iodine formed during the reaction is excluded based on the results of the control experiment with unmodified insulin and tyrosine-based selenonium salt 15, in which iodo-insulin was not observed and unmodified insulin was recovered in 86% yield (Supplementary Fig. 64). Our method provides an alternative synthesis approach to insulin with 3-iodo-Y26 in addition to native chemical ligation9. Bromo-ubiquitin 27 was obtained in 71% yield and its secondary structure matches that of the native ubiquitin upon renaturation, as judged by circular dichroism spectroscopy (Supplementary Fig. 68).
Conclusions
We report the design and utility of a selenoxide that can achieve site-selective single-atom modifications on tyrosine residues. The method represented an approach for C–H functionalization of peptides and begins to tackle C–H functionalization of proteins with small structural changes. Although the limitations imposed by the reactivity and reaction conditions, for example, pH 3, does not yet allow selective functionalization of most proteins, the method introduces a concept that allows for the introduction of a versatile linchpin by selective C–H functionalization of proteins, including single-atom modifications that are currently not readily achieved otherwise.
Methods
Initial study on functionalization of compound 2
Preparation of stock solutions of sulfoxides
Under ambient atmosphere, a scintillation vial (4 ml) equipped with a Teflon-coated magnetic stir bar was charged with sulfoxide (0.050 mmol) and the solvent MeCN (2.0 mL, 25 mM). The mixture was stirred at 25 °C until all the solids dissolved. The resulting solution was stored at 25 °C and be directly used for reactions without any further operations.
Preparation of stock solutions of selenoxides
Under ambient atmosphere, a scintillation vial (4 ml) was charged with selenoxide (0.050 mmol, final concentration 25 mM) and 2.0 ml of an aqueous H3PO4 solution (50 mM) in ultrahigh-quality (UHQ)-H2O. The suspension was heated at 90 °C for 30 min to give a yellow solution. After cooling to 25 °C, the resulting solution was stored in the dark at 25 °C and directly used for reactions without any further operations.
Reaction set-up
Under ambient atmosphere, a gas chromatography (GC) vial (2 ml) equipped with a Teflon-coated mini magnetic stir bar was charged with 100 µl of sodium citrate buffer (pH 3.0, 500 mM, final concentration 100 mM) or 100 µl of an H2SO4 stock solution (250 mM, final concentration 50 mM, final pH 1.0) in UHQ-H2O, 250 µl of UHQ-H2O and 100 µl of the sulfoxide stock solution (25 mM, 2.5 µmol, 2.0 equiv., final concentration 5.0 mM) in MeCN (final volume percentage 20%) or 100 µl of the selenoxide stock solution (25 mM, 2.5 µmol, 2.0 equiv., final concentration 5.0 mM) in H3PO4 solution (50 mM) in UHQ-H2O. The mixture was stirred at 30 °C for 5 min followed by the addition of 50 µl of a NAc-Tyr-NH2 2 stock solution (25 mM, 1.25 µmol, 0.28 mg, 1.0 equiv., final concentration 2.5 mM) in UHQ-H2O. Then the mixture was stirred at 30 °C for 18 h, after which 25 µl of a NaOH stock solution (2.0 M, 50 µmol, 2.0 mg, 40 equiv.) in UHQ-H2O was introduced to the reaction in aqueous H2SO4 solution, and 56 µl of a NaOH stock solution (2.0 M, 0.11 mmol, 4.5 mg, 90 equiv.) in UHQ-H2O was introduced to the reaction in sodium citrate buffer. The mixture was then concentrated to dryness under reduced pressure, the residue was dissolved in CD3OD (∼0.5 ml) and 5.0 µl of a CH2Br2 solution (0.50 M, 0.43 mg, 2.5 µmol, 2.0 equiv) in CD3OD was added as internal standard. The mixture was analysed by 1H NMR spectroscopy and a singlet peak at 5.16 ppm (CH2Br2) was set as 4.00. The yields of the different selenonium salts were determined as follows:
Yield of the product of 4 = integration of peak (δ 6.46 ppm (d, 1H)) × 100%.
Yield of the product of 5 = integration of peak (δ 6.09 ppm (d, 1H)) × 100%.
Yield of the product of 6 = integration of peak (δ 6.15 ppm (d, 1H)) × 100%.
Yield of the product of 1 = integration of peak (δ 6.11 ppm (d, 1H)) × 100%.
General procedure for NMR study
Under ambient atmosphere, a GC vial (2 ml) equipped with a Teflon-coated mini magnetic stir bar was charged with selenoxide (0.010 mmol, final concentration 20 mM), UHQ-H2O (225 µl) and MeCN-d3 (250 µl, final volume percentage 50%). For the control experiment in Fig. 2g, the MeCN-d3 was replaced by a solution of oxazole (40 mM, 250 µl, 0.010 mmol, 0.69 mg, final concentration 20 mM) in MeCN-d3 (final volume percentage 50%). The pH of the mixture was adjusted by the addition of a solution of H2SO4 (1.0 M, 25 µl, 25 µmol, 2.5 mg, 2.5 equiv., final concentration 50 mM, pH 1) in UHQ-H2O or a solution of NaOH (2.0 M, 25 µl, 50 µmol, 2.0 mg, 5.0 equiv., final concentration 0.10 M, pH 13) in UHQ-H2O. The mixture was stirred at 25 °C until all the solids dissolved. The mixtures were analysed by 1H NMR, 13C NMR, 77Se NMR and 1H–15N HMBC (heteronuclear multiple bond correlation) spectroscopy.
General protocol for selenium modification of peptides and proteins
Preparation of the stock solutions of the selenoxide 1
Under ambient atmosphere, a scintillation vial (4 ml) was charged with the selenoxide 1 (20 mg, 0.050 mmol, final concentration 50 mM) and 1.0 ml of a H3PO4 solution (0.10 M) in UHQ-H2O. The suspension was heated at 90 °C for 30 min to give a yellow solution. After cooling to 25 °C, the resulting solution was stored in the dark at 25 °C and used directly for reactions without any further operations.
Preparation of the stock solutions of peptide and protein starting materials
All peptides and proteins were directly used without any further purification. The peptides and proteins were dissolved in UHQ-H2O and the solution concentration was determined based on the ultraviolet (UV) absorption at 280 nm measured by Nanodrop (average of three measurements). With the molecular weight (average isotopic mass) and the corresponding extinction coefficient at 280 nm, which are calculated based on the amino acid sequence (https://web.expasy.org/protparam), the concentration of the peptide or protein solution can be calculated as follows:
The solution was then diluted to a certain concentration (typically, 0.5–2.0 mM) by UHQ-H2O and stored at 4 °C.
Reaction set-up
At 20–25 °C, NaPi buffer (pH 3.0) or an aqueous solution of TFA (for insulin modification) and the necessary additives depending on the starting material (Fig. 3 and Supplementary Information, pages 89–120) were added to an Eppendorf tube (1.5 ml). The resulting mixture was diluted to 70–85 µl by UHQ-H2O followed by the addition of 10 µl of a stock solution of the reagent 1 (50 mM, 0.50 µmol, 50 or 100 equiv., final concentration 5.0 mM) in phosphoric acid solution (0.10 M) in UHQ-H2O. The mixture was vortexed for 5 s, transferred into a Thermocycler preheated at 30 °C (or 37 °C) and incubated at 30 °C (or 37 °C) for 30 min at 600 rpm. Next, 5–10 µl of the stock solution of peptide starting material (1.0–2.0 mM, 10 nmol, 1.0 equiv., final concentration 0.10 mM) in UHQ-H2O or 10–20 µl of protein starting material (0.25–0.50 mM, 5.0 nmol, 1.0 equiv., final concentration 50 µM) in UHQ-H2O was added at 20–25 °C (note that the total volume of the reaction is 100 µl). The mixture was vortexed for 5 s, transferred into a Thermocycler preheated at 30 °C (or 37 °C), and incubated at 30 °C (or 37 °C) for 12–16 h at 600 rpm. Then, excess selenoxide was removed either by reduction with sodium sulfite followed by extraction with ethyl acetate (for example, Supplementary Information, page 89), or by ultrafiltration with Amicon Ultra Centrifugal Filters (Supplementary Information, page 111). The resulting mixture was subjected to liquid chromatography–mass spectrometry (LC–MS) analysis and the yield was determined by UV absorption (λmax = 328 nm). For multisite modification (11 and 13), yields and ratios were determined by UV absorption at 328 nm and deconvoluted zero-charge mass spectra analysis (Supplementary Information, pages 101–102, 113).
General protocol for conversions of the selenonium salt 15 by photomediated reactions
Preparation of oxygen-free buffers and stock solutions
A scintillation vial (4 ml) was charged with the buffer or the stock solution (2 ml). An argon flow was gently passed through the solution via a needle (0.80 mm diameter × 120 mm) for 3 min. Then, the vial was closed by a screw cap and stored under a nitrogen atmosphere (in a glovebox) for further transformations.
Reaction set-up
Under a nitrogen atmosphere (it is recommended to perform these reactions in a glovebox), a GC vial (2 ml) equipped with a Teflon-coated mini magnetic stir bar was charged with the necessary reductants and reagents depending on the transformation (Fig. 4a and Supplementary Information, pages 68–78), followed by the addition of buffer (final concentration 100 mM) in UHQ-H2O, a stock solution of selenonium salt 15 (16 mM, 20 µl, 0.32 µmol, 0.23 mg, 1.0 equiv., final concentration 1.6 mM) in MeCN/UHQ-H2O (1/1, v/v). The cosolvent MeCN was added to reach a final volume percentage of 20% or 40% (v/v), and UHQ-H2O was added to reach the final volume of 200 µl. The vial was closed by a screw cap, placed between two Kessil PR160 390-nm light-emitting diodes (LEDs) (2.5 cm away from each lamp) and irradiated for 10–20 min; the temperature of the reaction mixture was kept at approximately 30 °C through the use of a cooling fan. Then (note the oxidation step of the hydroxylation transformation and the ring-closure step of the coumarin formation; see Supplementary Information, pages 75 and 77, respectively), the mixture was diluted with 800 µl of MeCN/UHQ-H2O (1/1, v/v) and saturated with anhydrous Na2SO4 (∼0.3 g). The mixture was extracted by tetrahydrofuran/ethyl acetate (1/1, v/v, 500 µl × 3) and the organic layers were combined, concentrated under reduced pressure and dried in vacuo. The residue was dissolved in CD3OD (∼0.5 ml), and 2.5 µl of a 1,3,5-trimethoxybenzene solution (0.10 M, 0.25 µmol, 42 µg, 0.78 equiv.) in CD3OD was added as internal standard. The mixture was analysed by 1H NMR and a singlet peak at 6.08 ppm (1,3,5-trimethoxybenzene) was set as 3.00 for the calculation of the product yield.
General protocol for conversions of selenium-modified peptides and proteins by photomediated reactions
Under a nitrogen atmosphere (it is recommended to perform these reactions in a glovebox) and at 20–25 °C, 10–20 µl of the selenium bioconjugate solution (that is, the product mixture of the corresponding selenium modification step) and the necessary reagents, the additives and the cosolvent MeCN, depending on the transformation (Fig. 4b and Supplementary Information, pages 124–149), were added to an Eppendorf tube (1.5 ml). The concentration of the starting material was controlled in the range of 5–40 µM, and the volume of the reaction was controlled in the range of 45–50 µl. Then, the mixture was vortexed for 5 s (for 11 and 12, the mixture was vortexed for 5 s and transferred into a Thermocycler preheated at 25 °C and incubated at 25 °C for 5 min at 600 rpm). The Eppendorf tube was floated on an ice-water bath under one Kessil PR160 390-nm LED (2.5 cm away from the LED; Supplementary Fig. 53) and irradiated for 15–20 min. The temperature of the reaction mixture was kept at approximately 0 °C through the use of the ice-water bath. Then, the reaction was worked up according to the transformation (Supplementary Information, pages 124–149), and the resulting mixture was subjected to LC–MS analysis; the yield was determined by UV absorption (λmax = 214 nm). For protein modifications (26 and 27), yields and ratios were determined by UV absorption at 214 nm and deconvoluted zero-charge mass spectra analysis (Supplementary Information, page 122).
Protocol for Suzuki coupling of 15 and selenium-modified bivalirudin 8
For selenonium salt 15
Under an ambient atmosphere, a scintillation vial (4 ml) equipped with a Teflon-coated mini magnetic stir bar was charged with the coupling partner (4-fluorophenyl)boronic acid (2.8 mg, 20 µmol, 20 equiv.) and the catalyst Pd(OAc)2 (1.1 mg, 5.0 µmol, 5.0 equiv.), followed by the addition of NaPi buffer (pH 8.0, 200 mM, 500 µl, final concentration 100 mM) and a stock solution of selenonium salt 15 (25 mM, 40 µl, 1.0 µmol, 0.71 mg, 1.0 equiv., final concentration 1.0 mM) in DMSO/UHQ-H2O (1/1, v/v), UHQ-H2O (380 µl). The mixture was stirred at 37 °C for 5 min followed by the addition of the cosolvent DMSO (80 µl, final volume percentage 10%). Then the mixture was stirred at 37 °C for 2 h, after which it was diluted with methanol (0.80 ml) and filtered using a 0.22-µm filter. The filtrate was concentrated under reduced pressure and dried in vacuo. The residue was dissolved in CD3OD, and 10 µl of a 4-fluorobenzotrifluoride solution (0.10 M, 1.0 µmol, 0.16 mg, 1.0 equiv.) in CD3OD was added as internal standard. The mixture was analysed by 19F NMR to determine the yield of the product (75%; for details of yield determination, see Supplementary Information, page 78).
For selenium bioconjugate 8
(For preparation of the catalyst stock solution, see Supplementary Information, pages 130 and 131.) Under a nitrogen atmosphere (in a glovebox) and at 20–25 °C, 4.0 µl of a p-F-phenylboronic acid stock solution (0.20 M, 0.80 µmol, 0.11 mg, 4.7 × 102 equiv., final concentration 19 mM) in DMA and 8.0 µl of the dilution buffer (NaPi buffer, pH 8.0, 0.25 M, containing DMA 25% (v/v)) were added to an Eppendorf tube (1.5 ml). Then, 2.0 µl of a NaOH stock solution (1.5 M, 3.0 µmol, 0.12 mg, final concentration 0.11 M) in UHQ-H2O was added for the neutralization of the bivalirudin–selenonium conjugate 8 stock solution added in the next step. The mixture was vortexed for 5 s, followed by the addition of 20 µl of a bivalirudin–selenonium conjugate 8 stock solution (84 µM, 1.7 nmol, 4.3 µg, 1.0 equiv., final concentration 40 µM) in NaPi buffer (pH 3.0, 0.20 M). Next, 8.0 µl of the stock solution of palladium catalyst (Pd(OAc)2, 13 mM, 0.10 µmol, 22 µg, 59 equiv., final concentration 2.4 mM; TPPTS, 25 mM, 0.20 µmol, 0.11 mg, 1.2 × 102 equiv., final concentration 4.8 mM) in the dilution buffer was added to finish the preparation of the reaction (the final concentration of NaPi buffer was 0.19 M, the final volume percentage of DMA was 19%, and the final solution pH was 8–9, as indicated by general pH test paper). The mixture was vortexed for 5 s again, transferred into a Thermocycler preheated at 37 °C, and incubated at 37 °C for 40 min at 600 rpm. Then, 40 µl of a sodium diethyldithiocarbamate trihydrate (DTC) stock solution (10 mM, 0.40 µmol, 90 µg, 4.0 equiv. to Pd(OAc)2) in UHQ-H2O was added and the mixture was vortexed for 5 s, transferred into a Thermocycler preheated at 25 °C, and incubated at 25 °C for 10 min at 600 rpm. After that, the mixture was centrifuged at 4 °C for 10 min at 21,694g. The pellets were discarded and the supernatant was stored at −20 °C, and was subjected to LC–MS analysis. The yield was determined by UV absorption (λmax = 214 nm) (78%, average of three experiments).
Crystallization of selenoxides
1H+HSO4‒·(MeOH)2 (CCDC 2291793): the crystal structure of 1H+HSO4‒·(MeOH)2 was obtained as follows. First, 1 (∼3 mg) was dissolved in a solution of H2SO4 (0.2 M) in methanol (∼0.1 ml) to afford a bright-yellow solution at 25 °C. Then, the solution was slowly added to an NMR tube filled with toluene (∼0.5 mL), and left standing in the dark at 4 °C for 24 h.
5H+MsO‒ (CCDC 2304770): the crystal structure of 5H+MsO‒ was obtained as follows. First, 5 (∼2 mg) was dissolved in a solution of methanesulfonic acid (0.5 M) in methanol (30 µl) at 25 °C in a GC vial (2 ml) to afford a pale yellow solution. Then, the solution was diluted with toluene (60 µl), followed by the slow addition of hexanes (∼0.4 ml) above the solution. (Note that it is important not to shake the vial during/after hexane addition.) The mixture was left standing in the dark at 25 °C for 24 h.
Data availability
The data reported in this paper are available within the article and its Supplementary Information. Source data for the calibration curves of peptidyl substrates and the UV–visible absorption spectrum of 15 are provided with this paper. Crystallographic data for the structure reported in this article have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC 2291793 (1H+HSO4‒·(MeOH)2) and 2304770 (5H+MsO‒). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
References
Johnson, L. N. & Lewis, R. J. Structural basis for control by phosphorylation. Chem. Rev. 101, 2209–2242 (2001).
Walsh, C. T., Garneau‐Tsodikova, S. & Gatto, G. J. Jr Protein posttranslational modifications: the chemistry of proteome diversifications. Angew. Chem. Int. Ed. 44, 7342–7372 (2005).
Jones, L. H., Narayanan, A. & Hett, E. C. Understanding and applying tyrosine biochemical diversity. Mol. Biosyst. 10, 952–969 (2014).
Sawant Dessai, A., Kalhotra, P., Novickis, A. T. & Dasgupta, S. Regulation of tumor metabolism by post translational modifications on metabolic enzymes. Cancer Gene Ther 30, 548–558 (2023).
Hinner, M. J. & Johnsson, K. How to obtain labeled proteins and what to do with them. Curr. Opin. Chem. Biol. 21, 766–776 (2010).
Shadish, J. A. & DeForest, C. A. Site-selective protein modification: from functionalized proteins to functional biomaterials. Matter 2, 50–77 (2020).
Fu, Z., Li, S., Han, S., Shi, C. & Zhang, Y. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct. Target. Ther. 7, 93 (2022).
Jakka, S. R., Govindaraj, V. & Mugesh, G. A single atom change facilitates the membrane transport of green fluorescent proteins in mammalian cells. Angew. Chem. Int. Ed. 58, 7713–7717 (2019).
El Hage, K. et al. Extending halogen-based medicinal chemistry to proteins. J. Biol. Chem. 291, 27023–27041 (2016).
Walsh, G. & Jefferis, R. Post-translational modifications in the context of therapeutic proteins. Nat. Biotechnol. 24, 1241–1252 (2006).
Hoyt, E. A., Cal, P. M., Oliveira, B. L. & Bernardes, G. J. Contemporary approaches to site-selective protein modification. Nat. Rev. Chem. 3, 147–171 (2019).
Dawson, P. et al. Synthesis of proteins by native chemical ligation. Science 266, 776–779 (1994).
Kulkarni, S. S. et al. Rapid and efficient protein synthesis through expansion of the native chemical ligation concept. Nat. Rev. Chem. 2, 0122 (2018).
Boutureira, O. & Bernardes, G. J. Advances in chemical protein modification. Chem. Rev. 115, 2174–2195 (2015).
Baslé, E., Joubert, N. & Pucheault, M. Protein chemical modification on endogenous amino acids. Chem. Biol. 17, 213–227 (2010).
Josephson, B. et al. Light-driven post-translational installation of reactive protein side chains. Nature 585, 530–537 (2020).
Fu, X. P. et al. Stereoretentive post-translational protein editing. ACS Cent. Sci. 9, 405–416 (2023).
Long, T. et al. Light-controlled tyrosine nitration of proteins. Angew. Chem. Int. Ed. 60, 13414–13422 (2021).
Luo, P. et al. Photochemical bromination and iodination of peptides and proteins by photoexcitation of aqueous halides. Chem. Commun. 57, 11972–11975 (2021).
Börgel, J. & Ritter, T. Late-stage functionalization. Chem 6, 1877–1887 (2020).
Guillemard, L., Kaplaneris, N., Ackermann, L. & Johansson, M. J. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 5, 522–545 (2021).
Zhang, L. & Ritter, T. A perspective on late-stage aromatic C–H bond functionalization. J. Am. Chem. Soc. 144, 2399–2414 (2022).
Echols, N. et al. Comprehensive analysis of amino acid and nucleotide composition in eukaryotic genomes, comparing genes and pseudogenes. Nucleic Acids Res. 30, 2515–2523 (2002).
Yoshikawa, S. et al. Redox-coupled crystal structural changes in bovine heart cytochrome c oxidase. Science 280, 1723–1729 (1998).
Elsasser, T. H., Li, C. J., Caperna, T. J., Kahl, S. & Schmidt, W. F. Growth hormone (GH)-associated nitration of Janus kinase-2 at the 1007Y-1008Y epitope impedes phosphorylation at this site: mechanism for and impact of a GH, AKT, and nitric oxide synthase axis on GH signal transduction. Endocrinology 148, 3792–3802 (2007).
Ito, N. et al. Novel thioether bond revealed by a 1.7 Å crystal structure of galactose oxidase. Nature 350, 87–90 (1991).
Pandyarajan, V. et al. Biophysical optimization of a therapeutic protein by nonstandard mutagenesis. J. Biol. Chem. 289, 23367–23381 (2014).
Joshi, N. S., Whitaker, L. R. & Francis, M. B. A three-component Mannich-type reaction for selective tyrosine bioconjugation. J. Am. Chem. Soc. 126, 15942–15943 (2004).
Seim, K. L., Obermeyer, A. C. & Francis, M. B. Oxidative modification of native protein residues using cerium(IV) ammonium nitrate. J. Am. Chem. Soc. 133, 16970–16976 (2011).
Li, B. X. et al. Site-selective tyrosine bioconjugation via photoredox catalysis for native-to-bioorthogonal protein transformation. Nat. Chem. 13, 902–908 (2021).
Zhao, Z., Shimon, D. & Metanis, N. Chemoselective copper-mediated modification of selenocysteines in peptides and proteins. J. Am. Chem. Soc. 143, 12817–12824 (2021).
Cohen, D. T. et al. A chemoselective strategy for late-stage functionalization of complex small molecules with polypeptides and proteins. Nat. Chem. 11, 78–85 (2019).
Flood, D. T., Yan, N. L. & Dawson, P. E. Post-translational backbone engineering through selenomethionine-mediated incorporation of Freidinger lactams. Angew. Chem. Int. Ed. 57, 8697–8701 (2018).
Berger, F. et al. Site-selective and versatile aromatic C–H functionalization by thianthrenation. Nature 567, 223–228 (2019).
Li, J. et al. Photoredox catalysis with aryl sulfonium salts enables site-selective late-stage fluorination. Nat. Chem. 12, 56–62 (2020).
Ni, S. et al. Nickel meets aryl thianthrenium salts: Ni(I)-catalyzed halogenation of arenes. J. Am. Chem. Soc. 145, 9988–9993 (2023).
Cabrera-Afonso, M. J., Granados, A. & Molander, G. A. Sustainable thioetherification via electron donor–acceptor photoactivation using thianthrenium salts. Angew. Chem. Int. Ed. 61, e202202706 (2022).
Shine, H. J. & Murata, Y. Kinetics and mechanism of the reaction of the thianthrene cation radical with water. J. Am. Chem. Soc. 91, 1872–1874 (1969).
Juliá, F. et al. High site selectivity in electrophilic aromatic substitutions: mechanism of C–H thianthrenation. J. Am. Chem. Soc. 143, 16041–16054 (2021).
Reich, H. J. & Hondal, R. J. Why nature chose selenium. ACS Chem. Biol. 11, 821–841 (2016).
Mousa, R., Notis Dardashti, R. & Metanis, N. Selenium and selenocysteine in protein chemistry. Angew. Chem. Int. Ed. 56, 15818–15827 (2017).
Zhao, Z., Laps, S., Gichtin, J. S. & Metanis, N. Selenium chemistry for spatio-selective peptide and protein functionalization. Nat. Rev. Chem. 8, 211–229 (2024).
Arai, K., Dedachi, K. & Iwaoka, M. Rapid and quantitative disulfide bond formation for a polypeptide chain using a cyclic selenoxide reagent in an aqueous medium. Chem. Eur. J. 17, 481–485 (2011).
Oliveira, V., Cremer, D. & Kraka, E. The many facets of chalcogen bonding: described by vibrational spectroscopy. J. Phys. Chem. A 121, 6845–6862 (2017).
Selvakumar, K. & Singh, H. B. Adaptive responses of sterically confined intramolecular chalcogen bonds. Chem. Sci. 9, 7027–7042 (2018).
He, X., Wang, X., Tse, Y. L., Ke, Z. & Yeung, Y. Y. Applications of selenonium cations as Lewis acids in organocatalytic reactions. Angew. Chem. Int. Ed. 57, 12869–12873 (2018).
Bondi, A. V. van der Waals volumes and radii. J. Phys. Chem. 68, 441–451 (1964).
Live, D. H., Davis, D. G., Agosta, W. C. & Cowburn, D. Long range hydrogen bond mediated effects in peptides: nitrogen-15 NMR study of gramicidin S in water and organic solvents. J. Am. Chem. Soc. 10, 1939–1941 (1984).
Semenov, V. A., Samultsev, D. O. & Krivdin, L. B. Theoretical and experimental study of 15N NMR protonation shifts. Magn. Reson. Chem. 53, 433–441 (2015).
Day, R. O. & Holmes, R. R. Crystal structures of a tetraoxy spirocyclic selenurane and tellurane. Lone pair effects. Inorg. Chem. 20, 3071–3075 (1981).
Zhao, Y., Yu, C., Liang, W., Atodiresei, I. L. & Patureau, F. W. TEMPO-mediated late stage photochemical hydroxylation of biaryl sulfonium salts. Chem. Commun. 58, 2846–2849 (2022).
Galli, C. Radical reactions of arenediazonium ions: an easy entry into the chemistry of the aryl radical. Chem. Rev. 88, 765–792 (1988).
Ferrie, J. J. et al. Multicolor protein FRET with tryptophan, selective coumarin-cysteine labeling, and genetic acridonylalanine encoding. Chem. Commun. 53, 11072–11075 (2017).
Acknowledgements
We thank E. de Pedro Beato, J. Mateos, L. Zhang and Q. Cheng for helpful discussions. We thank J. Rust and C. Lehmann for X-ray analysis and discussion, and D. Kampen for mass spectrometry and high-resolution mass spectrometry–tandem mass spectrometry analysis. We thank the MPI für Kohlenforschung for funding. T.R. received the funding and the funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Funding
Open access funding provided by Max Planck Society.
Author information
Authors and Affiliations
Contributions
S.L. developed reagent 1. P.H. and J.M.M optimized the synthesis of reagents. M.L. obtained and analysed the NMR data. S.L. developed the bioconjugation and the transformations of selenonium salts. S.L. and M.H. performed the reactions on tyrosine derivatives, peptides and proteins. S.L., P.H., S.K., M.S.S., A.V. and H.H. obtained the LC–MS and LC–MS/MS data. S.L., P.H., M.S.S., A.V. and M.H. analysed the MS data. M.H. and J.S.-G. obtained and analysed the circular dichroism spectrometry data. F.B. and J.L. discovered the initial reactivity of selenoxides. S.L. and T.R. wrote the paper. T.R. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–76, Tables 1–41, experimental procedures, DFT calculation data, X-ray crystallographic analysis and product characterization.
Supplementary Data 1
Crystallographic data for compound 1H+HSO4–·(MeOH)2; CCDC reference 2291793.
Supplementary Data 2
Crystallographic data for compound for 5H+MsO–; CCDC reference 2304770.
Supplementary Data 3
Numerical source data for Supplementary Figs. 32, 33, 35 and 37.
Supplementary Data 4
Numerical source data for Supplementary Fig. 70.
Source data
Source Data Fig. 2
Statistical source data for Fig. 2f.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, S., Hirao, M., Hartmann, P. et al. A selenoxide for single-atom protein modification of tyrosine residues enabled by water-resistant chalcogen and hydrogen bonding. Nat. Chem. 17, 1331–1339 (2025). https://doi.org/10.1038/s41557-025-01842-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01842-8
This article is cited by
-
Selenoxides unlock C–H bonds of biomacromolecules
Nature Chemistry (2025)
-
On-DNA C–H functionalization of electron-rich arenes for DNA-encoded libraries
Nature Chemistry (2025)