Abstract
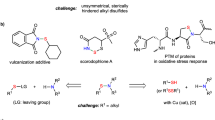
Functional group interconversions are particularly sought after by medicinal chemists as a means to enable both lead optimization and library diversification. Here we report SO2 insertion into the C–N bond of primary amines, enabling the direct synthesis of primary sulfonamides without preactivation and effectively inverting the nitrogen’s properties (acidity, hydrogen bonding and so on). The key to this transformation is the implementation of an anomeric amide as a dual-function reagent that both serves to cleave the initial C–N bond and delivers a nitrogen atom to the product after SO2 incorporation. The process tolerates a wide array of functionalities and can be run in an automated fashion, thus allowing libraries of amines to be viable progenitors to highly desirable sulfonamides. Mechanistic studies support an isodiazene radical chain mechanism that generates an intermediate sulfinate that reacts with the anomeric amide to forge the S–N bond. Our protocol was used to conduct a high-throughput library diversification campaign, was applied to the synthesis and modification of approved active pharmaceutical ingredients and was used to enable a net CO-to-SO2 isosteric replacement approach.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the Supplementary Information.
References
Ertl, P., Altmann, E. & McKenna, J. M. The most common functional groups in bioactive molecules and how their popularity has evolved over time. J. Med. Chem. 63, 8408–8418 (2020).
Bhutani, P. et al. U.S. FDA approved drugs from 2015–June 2020: a perspective. J. Med. Chem. 64, 2339–2381 (2021).
Younis, Y. et al. Structure–activity-relationship studies around the 2-amino group and pyridine core of antimalarial 3,5-diarylaminopyridines lead to a novel series of pyrazine analogues with oral in vivo activity. J. Med. Chem. 56, 8860–8871 (2013).
Zhang, S. et al. A small-molecule activation mechanism that directly opens the KCNQ2 channel. Nat. Chem. Biol. 20, 847–856 (2024).
Cohen, L. J. et al. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature 549, 48–53 (2017).
Velilla, J. A. et al. Structural basis of colibactin activation by the ClbP peptidase. Nat. Chem. Biol. 19, 151–158 (2023).
Flinspach, M. L. et al. Structural basis for dipeptide amide isoform-selective inhibition of neuronal nitric oxide synthase. Nat. Struct. Mol. Biol. 11, 54–59 (2004).
Lopez, M. et al. S-Glycosyl primary sulfonamides—a new structural class for selective inhibition of cancer-associated carbonic anhydrases. J. Med. Chem. 52, 6421–6432 (2009).
Uehara, T. et al. Selective degradation of splicing factor CAPERα by anticancer sulfonamides. Nat. Chem. Biol. 13, 675–680 (2017).
Gunaga, P. et al. Selective IKur inhibitors for the potential treatment of atrial fibrillation: optimization of the phenyl quinazoline series leading to clinical candidate 5-[5-phenyl-4-(pyridin-2-ylmethylamino)quinazolin-2-yl]pyridine-3-sulfonamide. J. Med. Chem. 60, 3795–3803 (2017).
Ovung, A. & Bhattacharyya, J. Sulfonamide drugs: structure, antibacterial property, toxicity, and biophysical interactions. Biophys. Rev. 13, 259–272 (2021).
Scott, K. A. & Njardarson, J. T. Analysis of US FDA-approved drugs containing sulfur atoms. Top. Curr. Chem. 376, 5 (2018).
Mujumdar, P. & Poulsen, S.-A. Natural product primary sulfonamides and primary sulfamates. J. Nat. Prod. 78, 1470–1477 (2015).
Plunkett, S., Basch, C. H., Santana, S. O. & Watson, M. P. Harnessing alkylpyridinium salts as electrophiles in deaminative alkyl–alkyl cross-couplings. J. Am. Chem. Soc. 141, 2257–2262 (2019).
Kiss, R., Sandor, M. & Szalai, F. A. http://Mcule.com: a public web service for drug discovery. J. Cheminformatics 4, P17 (2012).
Pedersen, P. S., Blakemore, D. C., Chinigo, G. M., Knauber, T. & MacMillan, D. W. C. One-pot synthesis of sulfonamides from unactivated acids and amines via aromatic decarboxylative halosulfonylation. J. Am. Chem. Soc. 145, 21189–21196 (2023).
Andrews, J. A. et al. Photocatalytic carboxylate to sulfinamide switching delivers a divergent synthesis of sulfonamides and sulfonimidamides. J. Am. Chem. Soc. 145, 21623–21629 (2023).
Zhuang, Z. et al. Visible-light-induced decarboxylative aminosulfonylation of (hetero)aryl carboxylic oxime esters. Org. Lett. 26, 713–718 (2024).
Zong, Z. et al. Conversion of carboxylic acids to sulfonamide bioisosteres via energy transfer photocatalysis. Org. Lett. 26, 8626–8631 (2024).
Carson, W. P. II, Sarver, P. J., Goudy, N. S. & MacMillan, D. W. C. Photoredox catalysis-enabled sulfination of alcohols and bromides. J. Am. Chem. Soc. 145, 20767–20774 (2023).
Li, Y., Xiao, F., Guo, Y. & Zeng, Y. Recent developments in deaminative functionalization of alkyl amines. Eur. J. Org. Chem. 2021, 1215–1228 (2021).
Berger, K. J. & Levin, M. D. Reframing primary alkyl amines as aliphatic building blocks. Org. Biomol. Chem. 19, 11–36 (2021).
Kong, D., Moon, P. J. & Lundgren, R. J. Radical coupling from alkyl amines. Nat. Catal. 2, 473–476 (2019).
Zhao, F. et al. Photocatalytic hydrogen-evolving cross-coupling of arenes with primary amines. Org. Lett. 20, 7753–7757 (2018).
Capperucci, A. & Tanini, D. Synthesis of nitroarenes by oxidation of aryl amines. Chemistry 4, 77–97 (2022).
Andrews, J. A., Pantaine, L. R. E., Palmer, C. F., Poole, D. L. & Willis, M. C. Sulfinates from amines: a radical approach to alkyl sulfonyl derivatives via donor–acceptor activation of pyridinium salts. Org. Lett. 23, 8488–8493 (2021).
Barton, D. H. R., Bringman, G., Lamotte, G., Hay Motherwell, R. S. & Motherwell, W. B. Radical deamination reactions of relevance to aminoglycoside chemistry. Tetrahedron Lett. 20, 2291–2294 (1979).
Wang, M., Fan, Q. & Jiang, X. Metal-free construction of primary sulfonamides through three diverse salts. Green Chem. 20, 5469–5473 (2018).
Ashley, M. A. & Rovis, T. Photoredox-catalyzed deaminative alkylation via C–N bond activation of primary amines. J. Am. Chem. Soc. 142, 18310–18316 (2020).
Pincekova, L., Merot, A., Schäfer, G. & Willis, M. C. Sandmeyer chlorosulfonylation of (hetero)aromatic amines using DABSO as an SO2 surrogate. Org. Lett. 26, 5951–5955 (2020).
Kennedy, S. H., Dherange, B. D., Berger, K. J. & Levin, M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 593, 223–227 (2021).
Masson-Makdissi, J. et al. Evidence for dearomatizing spirocyclization and dynamic effects in the quasi-stereospecific nitrogen deletion of tetrahydroisoquinolines. J. Am. Chem. Soc. 146, 17719–17727 (2024).
Berger, K. J. et al. Direct deamination of primary amines via isodiazene intermediates. J. Am. Chem. Soc. 143, 17366–17373 (2021).
Dherange, B. D. et al. Direct deaminative functionalization. J. Am. Chem. Soc. 145, 17–24 (2023).
Xue, J.-H., Li, Y., Liu, Y., Li, Q. & Wang, H. Site-specific deaminative trifluoromethylation of aliphatic primary amines. Angew. Chem. Int. Ed. 63, e202319030 (2024).
Good, A. & Thynne, J. C. J. Reaction of free radicals with sulphur dioxide. Part 2.—Ethyl radicals. Trans. Faraday Soc. 63, 2720–2727 (1967).
Sarver, P. J., Bissonnette, N. B. & MacMillan, D. W. C. Decatungstate-catalyzed C(sp3)–H sulfinylation: rapid access to diverse organosulfur functionality. J. Am. Chem. Soc. 143, 9737–9743 (2021).
Graham, S. L. & Scholz, T. H. The reaction of sulfinic acid salts with hydroxylamine-O-sulfonic acid. A useful synthesis of primary sulfonamides. Synthesis 1986, 1031–1032 (2002).
Emmett, E. J. & Willis, M. C. The development and application of sulfur dioxide surrogates in synthetic organic chemistry. Asian J. Org. Chem. 4, 602–611 (2015).
You, T., Zhang, M., Chen, J., Liu, H. & Xia, Y. Ruthenium(ii)-catalyzed reductive N–O bond cleavage of N-OR (R = H, alkyl, or acyl) substituted amides and sulfonamides. Org. Chem. Front. 8, 112–119 (2021).
Denisenko, A. et al. 2-Oxabicyclo[2.1.1]hexanes as saturated bioisosteres of the ortho-substituted phenyl ring. Nat. Chem. 15, 1155–1163 (2023).
Matošević, A. & Bosak, A. Carbamate group as structural motif in drugs: a review of carbamate derivatives used as therapeutic agents. Arch. Ind. Hyg. Toxicol. 71, 285–299 (2020).
De, S. et al. Pyridine: the scaffolds with significant clinical diversity. RSC Adv. 12, 15385–15406 (2022).
Digles, D. & Ecker, G. F. Self-organizing maps for in silico screening and data visualization. Mol. Inform. 30, 838–846 (2011).
van der Maaten, L. & Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008).
Walters, P. Visualizing chemical space. GitHub https://github.com/PatWalters/workshop/blob/master/predictive_models/2_visualizing_chemical_space.ipynb (2019).
Gualandi, A. et al. Photocatalytic radical alkylation of electrophilic olefins by benzylic and alkylic zinc-sulfinates. ACS Catal. 7, 5357–5362 (2017).
Lewis, N. L. et al. Phase I study of the safety, tolerability, and pharmacokinetics of oral CP-868,596, a highly specific platelet-derived growth factor receptor tyrosine kinase inhibitor in patients with advanced cancers. J. Clin. Oncol. 27, 5262–5269 (2009).
Galanis, A. et al. Crenolanib is a potent inhibitor of FLT3 with activity against resistance-conferring point mutants. Blood 123, 94–100 (2014).
Smith, C. C. et al. Crenolanib is a selective type I pan-FLT3 inhibitor. Proc. Natl Acad. Sci. USA 111, 5319–5324 (2014).
Ecker, A. K., Levorse, D. A., Victor, D. A. & Mitcheltree, M. J. Bioisostere effects on the EPSA of common permeability-limiting groups. ACS Med. Chem. Lett. 13, 964–971 (2022).
Hirayama, F. et al. Design, synthesis and biological activity of YM-60828 derivatives: potent and orally-bioavailable factor Xa inhibitors based on naphthoanilide and naphthalensulfonanilide templates. Bioorg. Med. Chem. 10, 2597–2610 (2002).
Loudon, G. M., Radhakrishna, A. S., Almond, M. R., Blodgett, J. K. & Boutin, R. H. Conversion of aliphatic amides into amines with [I,I-bis(trifluoroacetoxy)iodo]benzene. 1. Scope of the reaction. J. Org. Chem. 49, 4272–4276 (1984).
Evans, D. & Ripin, D. Evans pKa table. ACS Organic Division https://organicchemistrydata.org/hansreich/resources/pka/pka_data/evans_pKa_table.pdf (2024).
Neese, F., Hansen, A. & Liakos, D. G. Efficient and accurate approximations to the local coupled cluster singles doubles method using a truncated pair natural orbital basis. J. Chem. Phys. 131, 064103 (2009).
Riplinger, C., Pinski, P., Becker, U., Valeev, E. F. & Neese, F. Sparse maps—a systematic infrastructure for reduced-scaling electronic structure methods. II. Linear scaling domain based pair natural orbital coupled cluster theory. J. Chem. Phys. 144, 024109 (2016).
Acknowledgements
M.K. thanks the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (grant number RS-2023-00237898) for a postdoctoral fellowship. The research reported in this work was supported by a National Science Foundation CAREER Award (2235826) and by Johnson and Johnson Innovative Medicine. M.K. thanks the Korea Advanced Institute of Science and Technology (KAIST) and the Institute for Basic Science (IBS) for providing computational resources. We also thank S. Wolkenberg (Johnson & Johnson) for useful discussions and feedback.
Author information
Authors and Affiliations
Contributions
M.K., C.E.O. and C.B.K. designed and conducted experiments and collected and analysed the data. M.K. conducted the computational mechanistic evaluation. C.A.R. and C.G. conducted the high-throughput parallel synthesis experiments. J.C.R. conducted the docking study. M.D.L. and M.K. conceived of the project and wrote the manuscript with input from all authors. M.D.L. and C.B.K. supervised the research.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Nicholas Ball, Jie Wu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15, Supplementary Tables 1–8, Supplementary Discussion, and experimental procedures and spectra.
Supplementary Data
Computational data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kim, M., Obertone, C.E., Kelly, C.B. et al. Accessing sulfonamides via formal SO2 insertion into C–N bonds. Nat. Chem. 17, 1247–1255 (2025). https://doi.org/10.1038/s41557-025-01848-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01848-2
This article is cited by
-
Deaminative Giese-type reaction
Nature Chemistry (2025)
-
Stereoselective synthesis of (Z)-vinyl sulfonamides via kinetically controlled hydrosulfamoylation of alkynes
Science China Chemistry (2025)