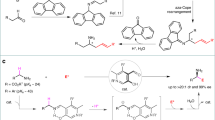
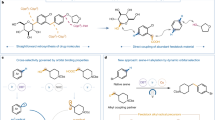
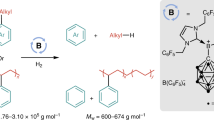
Abstract
The site-specific modification of amines has been a highly sought-after objective in organic synthesis. Despite the rapid advancement of carbon–hydrogen (C–H) bond functionalization methods, effective strategies for carbon–carbon (C–C) bond functionalization of amines remain elusive. Here we report a borane-catalysed method for the selective insertion of alkynes into alkyl C–C bonds of amines, resulting in the ring expansion of cyclic amines and chain elongation of acyclic amines. This approach begins with the cleavage of C–H bonds in amines, then transitioning to C–C bond functionalization upon reaction with alkynes. This method is effective with amines lacking an activating or leaving group and is suitable for late-stage functionalization of pharmaceuticals through C–C bond modification. Furthermore, by coupling this reaction with hydrolysis and hydrogenation steps, successive alkyne insertions are achieved, enabling modular and iterative alkyl growth of amines.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. All crystallographic data are available free of charge from the Cambridge Crystallographic Data Centre under CCDC numbers 2385045 (9), 2385038 (8g), 2385037 (8c), 2385036 (6o), 2385717 (14h), 2385715 (6d), 2417134 (6e), 2385041 (3z) and 2385040 (6b).
References
Jimenez, D. G., Poongavanam, V. & Kihlberg, J. Macrocycles in drug discovery—learning from the past for the future. J. Med. Chem. 66, 5377–5396 (2023).
Hussain, A., Yousufb, S. K. & Mukherjee, D. Importance and synthesis of benzannulated medium-sized and macrocyclic rings (BMRs). RSC Adv. 4, 43241–43257 (2014).
Driggers, E. M., Hale, S. P., Lee, J. & Terrett, N. K. The exploration of macrocycles for drug discovery—an underexploited structural class. Nat. Rev. Drug Discov. 7, 608–624 (2008).
Yet, L. Metal-mediated synthesis of medium-sized rings. Chem. Rev. 100, 2963–3007 (2000).
Gradillas, A. & Pérez-Castells, J. Macrocyclization by ring-closing metathesis in the total synthesis of natural products: reaction conditions and limitations. Angew. Chem. Int. Ed. 45, 6086–6101 (2006).
Mortensen, K. T., Osberger, T. J., King, T. A., Sore, H. F. & Spring, D. R. Strategies for the diversity-oriented synthesis of macrocycles. Chem. Rev. 119, 10288–10317 (2019).
Reyes, R. L., Iwai, T. & Sawamura, M. Construction of medium-sized rings by gold catalysis. Chem. Rev. 121, 8926–8947 (2021).
Otevrel, J., Eugui, M., Ričko, S. & Jørgensen, K. A. Enantioselective organocatalytic cycloadditions for the synthesis of medium-sized rings. Nat. Synth. 2, 1142–1158 (2023).
Rong, Z.-Q. et al. Nine-membered benzofuran-fused heterocycles: enantioselective synthesis by Pd-catalysis and rearrangement via transannular bond formation. J. Am. Chem. Soc. 139, 15304–15307 (2017).
Pan, J., Ho, T. O., Chen, Y.-C., Yang, B.-M. & Zhao, Y. Enantioselective construction of eight-membered N-heterocycles from simple 1,3-dienes via Pd(0) Lewis base catalysis. Angew. Chem. Int. Ed. 63, e202317703 (2024).
Donald, J. R. & Unsworth, W. P. Ring-expansion reactions in the synthesis of macrocycles and medium-sized rings. Chem. Eur. J. 23, 8780–8799 (2017).
Clarke, A. K. & Unsworth, W. P. A happy medium: the synthesis of medicinally important medium-sized rings via ring expansion. Chem. Sci. 11, 2876–2881 (2020).
Roque, J. B., Kuroda, Y., Göttemann, L. T. & Sarpong, R. Deconstructive fluorination of cyclic amines by carbon-carbon cleavage. Science 361, 171–174 (2018).
Roque, J. B., Kuroda, Y., Göttemann, L. T. & Sarpong, R. Deconstructive diversification of cyclic amines. Nature 564, 244–248 (2018).
Kennedy, S. H., Dherange, B. D., Berger, K. J. & Levin, M. D. Skeletal editing through direct nitrogen deletion of secondary amines. Nature 593, 223–227 (2021).
Jurczyk, J. et al. Photomediated ring contraction of saturated heterocycles. Science 373, 1004–1012 (2021).
Mendoza-Sanchez, R. et al. Cyclols revisited: facile synthesis of medium-sized cyclic peptides. Chem. Eur. J. 23, 13319–13322 (2017).
Hall, J. E., Matlock, J. V., Ward, J. W., Gray, K. V. & Clayden, J. Medium-ring nitrogen heterocycles through migratory ring expansion of metalated ureas. Angew. Chem. Int. Ed. 55, 11153–11157 (2016).
Lawer, A. et al. Internal nucleophilic catalyst mediated cyclisation/ring expansion cascades for the synthesis of medium-sized lactones and lactams. Angew. Chem. Int. Ed. 58, 13942–13947 (2019).
Zhao, W., Qian, H., Li, Z. & Sun, J. Catalytic ring expansion of cyclic hemiaminals for the synthesis of medium-ring lactams. Angew. Chem. Int. Ed. 54, 10005–10008 (2015).
Guney, T., Wenderski, T. A., Boudreau, M. W. & Tan, D. S. Synthesis of benzannulated medium-ring lactams via a tandem oxidative dearomatization-ring expansion reaction. Chem. Eur. J. 24, 13150–13157 (2018).
Li, L. et al. Radical aryl migration enables diversity-oriented synthesis of structurally diverse medium/macro- or bridged-rings. Nat. Commun. 7, 13852 (2016).
Wang, N. et al. Direct photocatalytic synthesis of medium-sized lactams by C−C bond cleavage. Angew. Chem. Int. Ed. 57, 14225–14229 (2018).
Zalessky, I. et al. A modular strategy for the synthesis of macrocycles and medium-sized rings via cyclization/ring expansion cascade reactions. J. Am. Chem. Soc. 146, 5702–5711 (2024).
Wu, L. et al. Diversified ring expansion of saturated cyclic amines enabled by azlactone insertion. Nat. Chem. 16, 1951–1959 (2024).
Millot, N., Santini, C. C., Fenet, B. & Basset, J. M.Formation and characterization of zwitterionic stereoisomers from the reaction of B(C6F5)3 and NEt2Ph: (E)- and (Z)-[EtPhN+=CHCH2-B−(C6F5)3]. Eur. J. Inorg. Chem. 20023328–3335 (2002).
Basak, S. et al. Electron deficient borane-mediated hydride abstraction in amines: stoichiometric and catalytic processes. Chem. Soc. Rev. 50, 3720–3737 (2021).
Saridakis, I., Klose, I., Jones, B. T. & Maulide, N. Hydride shuttle catalysis: from conventional to inverse mode. JACS Au 4, 3358–3369 (2024).
Shang, M. et al. C–H functionalization of amines via alkene-derived nucleophiles through cooperative action of chiral and achiral Lewis acid catalysts: applications in enantioselective synthesis. J. Am. Chem. Soc. 140, 10593–10601 (2018).
Maier, A. F. G. et al. Borane-catalyzed synthesis of quinolines bearing tetrasubstituted stereocenters by hydride abstraction-induced electrocyclization. Chem. Eur. J. 24, 16287–16291 (2018).
Tian, J.-J., Zeng, N.-N., Liu, N., Tu, X.-S. & Wang, X.-C. Intramolecular cyclizations of vinyl-substituted N,N‑dialkyl arylamines enabled by borane-assisted hydride transfer. ACS Catal. 9, 295–300 (2019).
Basak, S. et al. B(C6F5)3-catalyzed direct C3 alkylation of indoles and oxindoles. ACS Catal. 10, 4835–4840 (2020).
Zhang, J., Park, S. & Chang, S. Catalytic access to bridged sila-N-heterocycles from piperidines via cascade sp3 and sp2 C–Si bond formation. J. Am. Chem. Soc. 140, 13209–13213 (2018).
Fang, H., Xie, K., Kemper, S. & Oestreich, M. Consecutive β,β′-selective C(sp3)–H silylation of tertiary amines with dihydrosilanes catalyzed by B(C6F5)3. Angew. Chem. Int. Ed. 60, 8542–8546 (2021).
Chang, Y. et al. Enantioselective synthesis of N-alkylamines through β-amino C–H functionalization promoted by cooperative actions of B(C6F5)3 and a chiral Lewis acid co-catalyst. J. Am. Chem. Soc. 143, 2441–2455 (2021).
Klose, I., Mauro, G. D., Kaldre, D. & Maulide, N. Inverse hydride shuttle catalysis enables the stereoselective one-step synthesis of complex frameworks. Nat. Chem. 14, 1306–1310 (2022).
Zhang, M., Tang, Z.-L., Luo, H. & Wang, X.-C. β-C–H allylation of trialkylamines with allenes promoted by synergistic borane/palladium catalysis. Angew. Chem. Int. Ed. 63, e202317610 (2024).
Zhou, X.-Y. et al. B(C6F5)3‑catalyzed C(sp3)−H alkylation of tertiary amines with electron-deficient olefins: determinants of site selectivity. ACS Catal. 14, 8041–8049 (2024).
Liu, B., Romine, A. M., Rubel, C. Z., Engle, K. M. & Shi, B.-F. Transition-metal-catalyzed, coordination-assisted functionalization of nonactivated C(sp3)–H bonds. Chem. Rev. 121, 14957–15074 (2021).
Holmberg-Douglas, N. & Nicewicz, D. A. Photoredox-catalyzed C–H functionalization reactions. Chem. Rev. 122, 1925–2016 (2022).
Zhang, Z., Chen, P. & Liu, G. Copper-catalyzed radical relay in C(sp3)–H functionalization. Chem. Soc. Rev. 51, 1640–1658 (2022).
Mariano, P. S., Osborn, M. E., Dunaway-Mariano, D., Gunn, B. C. & Pettersen, R. C. The chemistry of azocines. Intermediates for the synthesis of pyrrolizidines. J. Org. Chem. 42, 2903–2910 (1977).
Takahata, H., Tomiguchi, A., Hagiwara, A. & Yamazaki, T. Activated lactams. VI. The cycloaddition reaction of cyclic ketene-S,N-acetals with dimethyl acetylenedicarboxylate. Chem. Pharm. Bull. 30, 3959–3964 (1982).
Reinhoudt, D. N. et al. Reactivity of cis-fused 3-(dialkylamino)cyclobutenes in polar and apolar solvents. Synthesis, X-ray structures, and reactions of cis,cis- and cis,trans-1,3-cycloalkadienes. J. Am. Chem. Soc. 106, 1341–1350 (1984).
Xu, G.-Q. et al. Dual C(sp3)–H bond functionalization of N-heterocycles through sequential visible-light photocatalyzed dehydrogenation/[2+2] cycloaddition reactions. Angew. Chem. Int. Ed. 57, 5110–5114 (2018).
Li, X. et al. Temperature-controlled divergent hydroamination cyclization [2+2]-cycloaddition cascade reactions of homopropargylic amines with 2-butynedioates: direct access to pyrrolo-b-cyclobutene and dihydro-1H-azepines. J. Org. Chem. 84, 1288–1298 (2019).
Efimov, I. V., Zhilyaev, D. I., Kulikova, L. N. & Voskressensky, L. G. Cycloaddition reactions of enamines. Eur. J. Org. Chem. 26, e202201450 (2023).
López, C. S., Faza, O. N. & de Lera, Á. R. Electrocyclic ring opening of cis-bicyclo[m.n.0]alkenes: the anti-Woodward–Hoffmann quest. Chem. Eur. J. 13, 5009–5017 (2007).
Guillemard, L., Kaplaneris, N., Ackermann, L. & Johansson, M. J. Late-stage C–H functionalization offers new opportunities in drug discovery. Nat. Rev. Chem. 5, 522–545 (2021).
Zhang, L. & Ritter, T. A perspective on late-stage aromatic C–H bond functionalization. J. Am. Chem. Soc. 144, 2399–2414 (2022).
Liang, Y.-F. et al. Carbon–carbon bond cleavage for late-stage functionalization. Chem. Rev. 123, 12313–12370 (2023).
Yu, X.-Y., Chen, J.-R. & Xiao, W.-J. Visible light-driven radical-mediated C–C bond cleavage/functionalization in organic synthesis. Chem. Rev. 121, 506–561 (2021).
Xia, Y. & Dong, G. Temporary or removable directing groups enable activation of unstrained C–C bonds. Nat. Rev. Chem. 4, 600–614 (2020).
Acknowledgements
We thank F. Wang for helpful discussions. We acknowledge support from National Natural Science Foundation of China (grant nos. 22461160280, 22371141, 22221002 and 22188101 to X.-C.W.), National Key R&D Program of China (grant no. 2021YFA1500200 to X.-C.W.), National Natural Science Foundation of China/RGC Joint Research Scheme (grant no. N_CUHK452-24 to H.L.) and China Postdoctoral Science Foundation (grant no. 2024M751519 to X.-Y.Z.).
Author information
Authors and Affiliations
Contributions
X.-C.W. conceived the concept and supervised the study. X.-Y.Z. performed the experiments and analysed the data. L.L. performed the DFT calculations. X.-C.W. and H.L. proposed the research direction and wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Alexander Pulis, Donghui Wei and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–7, Figs. 1–11, experimental procedures, computational details, data and spectra.
Supplementary Data 1
Crystallographic data for compound 3z; CCDC reference 2385041.
Supplementary Data 2
Crystallographic data for compound 6b; CCDC reference 2385040.
Supplementary Data 3
Crystallographic data for compound 6d; CCDC reference 2385715.
Supplementary Data 4
Crystallographic data for compound 6e; CCDC reference 2417134.
Supplementary Data 5
Crystallographic data for compound 6o; CCDC reference 2385036.
Supplementary Data 6
Crystallographic data for compound 8c; CCDC reference 2385037.
Supplementary Data 7
Crystallographic data for compound 8g; CCDC reference 2385038.
Supplementary Data 8
Crystallographic data for compound 9; CCDC reference 2385045.
Supplementary Data 9
Crystallographic data for compound 14h; CCDC reference 2385717.
Supplementary Data 10
Cartesian coordinates raw file for all computations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, XY., Liu, L., Lyu, H. et al. Modular alkyl growth in amines via the selective insertion of alkynes into C–C bonds. Nat. Chem. 17, 1323–1330 (2025). https://doi.org/10.1038/s41557-025-01849-1
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01849-1
This article is cited by
-
Ring expansion and chain extension of alkyl amines
Nature Chemistry (2025)