Abstract
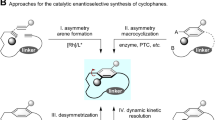
Dynamic kinetic resolution (DKR) is an efficient chiral induction strategy that transforms both enantiomers of the substrate racemates into the same optically enriched product. Typically, the absolute configurations of the products should be determined by the chiral catalyst. Here we report an adaptive DKR strategy that forges diverse azapolycycles with high stereochemical selectivities by taking full advantage of the dynamic interconversion of diastereomeric aminoalkyl cyclopalladated complexes. Notably, this innovative adaptive DKR model achieves alterations of absolute configurations at the contiguous stereocentres with the same chiral diphosphine-ligated palladium catalyst by changing the ring sizes of the annulation products. Moreover, a concise and efficient total synthesis of martinellic acid was carried out by using the adaptive DKR process as the key chirality forging and scaffold establishment step, which further demonstrated the efficacy of this strategy.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information. The supplementary crystallographic data for this article are available free of charge from the Cambridge Crystallographic Data Centre (CCDC) under accession numbers CCDC 2350635 (compound 4a), CCDC 2350636 (compound 3a), CCDC 2350637 (compound 5a), CCDC 2417704 (compound 5h), CCDC 2350638 (compound ent-6h), CCDC 2417682 (compound 7u), CCDC 2350639 (compound 7v) and CCDC 2350640 ([Pd(R)-MeOBIPHEP(OAc)2]).
References
Schneider, G., Neidhart, W., Giller, T. & Schmid, G. “Scaffold-hopping” by topological pharmacophore search: a contribution to virtual screening. Angew. Chem. Int. Ed. 38, 2894–2896 (1999).
Schneider, G. & Clark, D. E. Automated de novo drug design: are we nearly there yet? Angew. Chem. Int. Ed. 58, 10792–10803 (2019).
Sun, H., Tawa, G. & Wallqvist, A. Classification of scaffold-hopping approaches. Drug Discov. Today 17, 310–324 (2012).
Rafferty, R. J., Hicklin, R. W., Maloof, K. A. & Hergenrother, P. J. Synthesis of complex and diverse compounds through ring distortion of abietic acid. Angew. Chem. Int. Ed. 53, 220–224 (2014).
Hu, Y., Stumpfe, D. & Bajorath, J. Recent advances in scaffold hopping. J. Med. Chem. 60, 1238–1246 (2017).
Morcillo, S. P. Radical‐promoted C–C bond cleavage: a deconstructive approach for selective functionalization. Angew. Chem. Int. Ed. 58, 14044–14054 (2019).
Brotschi, C. et al. Oxadiazole derivatives as dual orexin receptor antagonists: synthesis, structure–activity relationships, and sleep-promoting properties in rats. ChemMedChem 14, 1257–1270 (2019).
Boss, C. et al. The quest for the best dual orexin receptor antagonist (daridorexant) for the treatment of insomnia disorders. ChemMedChem 15, 2286–2305 (2020).
Taylor, R. D., Maccoss, M. & Lawson, A. D. G. Rings in drugs. J. Med. Chem. 57, 5845–5859 (2014).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Xu, H., Zuend, S. J., Woll, M. G., Tao, Y. & Jacobsen, E. N. Asymmetric cooperative catalysis of strong brønsted acid-promoted reactions using chiral ureas. Science 327, 986–990 (2010).
Xie, Y. & List, B. Catalytic asymmetric intramolecular [4+2] cycloaddition of in situ generated ortho-quinone methides. Angew. Chem. Int. Ed. 56, 4936–4940 (2017).
Zhu, J.-X., Chen, Z.-C., Du, W. & Chen, Y.-C. Asymmetric auto-tandem palladium catalysis for 2,4-dienyl carbonates: ligand-controlled divergent synthesis. Angew. Chem. Int. Ed. 61, e202200880 (2022).
Liu, C. et al. Palladium-catalyzed cascade cyclization for the synthesis of fused benzo-aza-oxa-[5-6-5] tetracycles. Angew. Chem. Int. Ed. 61, e202215020 (2022).
Klose, I., Di Mauro, G., Kaldre, D. & Maulide, N. Inverse hydride shuttle catalysis enables the stereoselective one-step synthesis of complex frameworks. Nat. Chem. 14, 1306–1310 (2022).
Noyori, R., Tokunaga, M. & Kitamura, M. Stereoselective organic synthesis via dynamic kinetic resolution. Bull. Chem. Soc. Jpn. 68, 36–55 (1995).
Huerta, F. F., Minidis, A. B. E. & Bäckvall, J. E. Racemisation in asymmetric synthesis. Dynamic kinetic resolution and related processes in enzyme and metal catalysis. Chem. Soc. Rev. 30, 321–331 (2001).
Steinreiber, J., Faber, K. & Griengl, H. De‐racemization of enantiomers versus de-epimerization of diastereomers-classification of dynamic kinetic asymmetric transformations (DYKAT). Chem. Eur. J. 14, 8060–8072 (2008).
Pellissier, H. Chirality from Dynamic Kinetic Resolution (The Royal Society of Chemistry, 2011); https://doi.org/10.1039/9781849732673
Ruan, L.-X., Sun, B., Liu, J.-M. & Shi, S.-L. Dynamic kinetic asymmetric arylation and alkenylation of ketones. Science 379, 662–670 (2023).
DeHovitz, J. S. et al. Static to inducibly dynamic stereocontrol: the convergent use of racemic β-substituted ketones. Science 369, 1113–1118 (2020).
Qi, X., Liu, S. & Lan, Y. Computational studies on an aminomethylation precursor: (Xantphos)Pd(CH2NBn2)+. Organometallics 35, 1582–1585 (2016).
Yu, B., Zou, S., Liu, H. & Huang, H. Palladium-catalyzed ring-closing reaction via C–N bond metathesis for rapid construction of saturated N-heterocycles. J. Am. Chem. Soc. 142, 18341–18345 (2020).
Zhang, H., Jiang, T., Zhang, J. & Huang, H. Catalytic reactions directed by a structurally well-defined aminomethyl cyclopalladated complex. Acc. Chem. Res. 54, 4305–4318 (2021).
Zou, S., Zhao, Z. & Huang, H. Palladium‐catalyzed aminoalkylative cyclization enables modular synthesis of exocyclic 1,3-dienes. Angew. Chem. Int. Ed. 62, e202311603 (2023).
Cai, S., Zhao, Z., Yang, G. & Huang, H. Dynamic amine sorting enables multiselective construction of unsymmetrical chiral diamines. Nat. Chem. 16, 1972–1981 (2024).
Hamilton, J. Y., Rössler, S. L. & Carreira, E. M. Enantio- and diastereoselective spiroketalization catalyzed by chiral iridium complex. J. Am. Chem. Soc. 139, 8082–8085 (2017).
Zhang, G., Gao, B. & Huang, H. Palladium-catalyzed hydroaminocarbonylation of alkenes with amines: a strategy to overcome the basicity barrier imparted by aliphatic amines. Angew. Chem. Int. Ed. 54, 7657–7661 (2015).
Witherup, K. M. et al. Martinelline and martinellic acid, novel G-protein linked receptor antagonists from the tropical plant Martinella iquitosensis (Bignoniaceae). J. Am. Chem. Soc. 117, 6682–6685 (1995).
Haarr, M. B. & Sydnes, M. O. Synthesis of the hexahydropyrrolo-[3,2-c]-quinoline core structure and strategies for further elaboration to martinelline, martinellic acid, incargranine b, and seneciobipyrrolidine. Molecules 26, 341 (2021).
Ma, D., Xia, C., Jiang, J. & Zhang, J. First total synthesis of martinellic acid, a naturally occurring bradykinin receptor antagonist. Org. Lett. 3, 2189–2191 (2001).
Ikeda, S., Shibuya, M. & Iwabuchi, Y. Asymmetric total synthesis of martinelline and martinellic acid. Chem. Commun. 504–506 (2007).
Davies, S. G. et al. Asymmetric synthesis of (−)-martinellic acid. Org. Lett. 15, 2050–2053 (2013).
Pappoppula, M. & Aponick, A. Enantioselective total synthesis of (−)-martinellic acid. Angew. Chem. Int. Ed. 54, 15827–15830 (2015).
Acknowledgements
Financial support for this project was provided by the National Natural Science Foundation of China (grant nos. 21925111, 92356302 and 22350008 to H.H. and 22301290 to B.Y.), the Strategic Priority Research Program of Chinese Academy of Sciences (grant no. XDB0450301 to H.H.), the National Key R&D Program of China (grant no. 2023YFA1507500 to H.H.) and Anhui Provincial Natural Science Foundation (grant no. 2308085QB45 to B.Y.).
Author information
Authors and Affiliations
Contributions
H.H. conceived the concept and supervised the project. B.Y. and Y.H. developed the reaction and conducted the main experimental work. H.Z., B.Y. and H.H. prepared the paper, which was approved by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–21 and Tables 1–19.
Supplementary Data 1
X-ray structure of 3a.
Supplementary Data 2
X-ray structure of 4a.
Supplementary Data 3
X-ray structure of 5a.
Supplementary Data 4
X-ray structure of 5h.
Supplementary Data 5
X-ray structure of 7u.
Supplementary Data 6
X-ray structure of 7v.
Supplementary Data 7
X-ray structure of chiral Pd catalyst.
Supplementary Data 8
X-ray structure of ent-6h.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yu, B., Huang, Y., Zhang, H. et al. Adaptive dynamic kinetic resolution enables alteration of chiral induction with ring sizes. Nat. Chem. 17, 1256–1264 (2025). https://doi.org/10.1038/s41557-025-01850-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01850-8