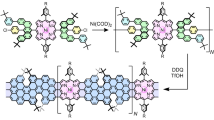
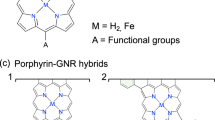
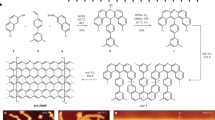
Abstract
Graphene nanoribbons (GNRs) with zigzag edges are promising materials for spintronic devices due to tunable bandgaps and spin-polarized edge states. Porphyrins offer complementary benefits such as desirable optoelectronic properties. Here we combine these features in a hybrid system by means of the on-surface synthesis of zigzag-edge GNRs embedded with porphyrins laterally fused along the ribbon backbone. Using scanning probe methods, we show that this design achieves strong electronic coupling between the porphyrin and the GNR. For transition metal porphyrins, pronounced exchange coupling between distant metal centres is mediated by the π-electron system. Such a hybrid d and π electron ribbon system introduces spin–orbit coupling and magnetic anisotropy to carbon nanomaterials, and holds great promise for coherent electrical control of electron spins.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data supporting the findings of this study are available within the article and Supplementary Information. The raw data supporting the results of the paper are available on the Materials Cloud repository63 at https://doi.org/10.24435/materialscloud:bw-ph. Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2281373 (2HPor–DBA), 2281312 (ZnPor–DBA) and 2391152 (FePorCl–DBA). Copies of the data can be obtained free of charge via www.ccdc.cam.ac.uk/structures/.
References
Chen, Z. et al. Synthesis of graphene nanoribbons by ambient-pressure chemical vapor deposition and device integration. J. Am. Chem. Soc. 138, 15488–15496 (2016).
Chen, C. et al. Sub-10-nm graphene nanoribbons with atomically smooth edges from squashed carbon nanotubes. Nat. Electron. 4, 653–663 (2021).
Talirz, L., Ruffieux, P. & Fasel, R. On-surface synthesis of atomically precise graphene nanoribbons. Adv. Mater. 28, 6222–6231 (2016).
Yang, X. et al. Two-dimensional graphene nanoribbons. J. Am. Chem. Soc. 130, 4216–4217 (2008).
Cai, J. et al. Atomically precise bottom-up fabrication of graphene nanoribbons. Nature 466, 470–473 (2010).
Clair, S. & de Oteyza, D. G. Controlling a chemical coupling reaction on a surface: tools and strategies for on-surface synthesis. Chem. Rev. 119, 4717–4776 (2019).
Ruffieux, P. et al. On-surface synthesis of graphene nanoribbons with zigzag edge topology. Nature 531, 489–492 (2016).
Gröning, O. et al. Engineering of robust topological quantum phases in graphene nanoribbons. Nature 560, 209–213 (2018).
Rizzo, D. J. et al. Topological band engineering of graphene nanoribbons. Nature 560, 204–208 (2018).
Turco, E. et al. Observation of the magnetic ground state of the two smallest triangular nanographenes. JACS Au 3, 1358–1364 (2023).
Mishra, S. et al. Topological frustration induces unconventional magnetism in a nanographene. Nat. Nanotechnol. 15, 22–28 (2020).
Mishra, S. et al. Observation of fractional edge excitations in nanographene spin chains. Nature 598, 287–292 (2021).
Gottfried, J. M. Surface chemistry of porphyrins and phthalocyanines. Surf. Sci. Rep. 70, 259–379 (2015).
Gilbert, W. et al. On-demand electrical control of spin qubits. Nat. Nanotechnol. 18, 131–136 (2023).
Nowack, K. C., Koppens, F. H. L., Nazarov, Y. V. & Vandersypen, L. M. K. Coherent control of a single electron spin with electric fields. Science 318, 1430–1433 (2007).
Baart, T. A., Fujita, T., Reichl, C., Wegscheider, W. & Vandersypen, L. M. K. Coherent spin-exchange via a quantum mediator. Nat. Nanotechnol. 12, 26–30 (2017).
Pozo, I. et al. Enhanced coherence by coupling spins through a delocalized π-system: vanadyl porphyrin dimers. Chem 10, 299–316 (2024).
Chen, Z. et al. Phase-coherent charge transport through a porphyrin nanoribbon. J. Am. Chem. Soc. 145, 15265–15274 (2023).
Kinikar, A. et al. On-surface synthesis of edge-extended zigzag graphene nanoribbons. Adv. Mater. 35, 2306311 (2023).
Gross, L., Mohn, F., Moll, N., Liljeroth, P. & Meyer, G. The chemical structure of a molecule resolved by atomic force microscopy. Science 325, 1110–1114 (2009).
Chen, Q. et al. Porphyrin-fused graphene nanoribbons. Nat. Chem. 16, 1133–1140 (2024).
Deng, J.-R., González, M. T., Zhu, H., Anderson, H. L. & Leary, E. Ballistic conductance through porphyrin nanoribbons. J. Am. Chem. Soc. 146, 3651–3659 (2024).
Li, Y., Jiang, X., Liu, Z. & Liu, Z. Strain effects in graphene and graphene nanoribbons: the underlying mechanism. Nano Res. 3, 545–556 (2010).
Xiang, F. et al. Planar π-extended cycloparaphenylenes featuring an all-armchair edge topology. Nat. Chem. 14, 871–876 (2022).
Paolesse, R., Nardis, S., Monti, D., Stefanelli, M. & Di Natale, C. Porphyrinoids for chemical sensor applications. Chem. Rev. 117, 2517–2583 (2017).
Liu, Z.-F. et al. Control of single-molecule junction conductance of porphyrins via a transition-metal center. Nano Lett. 14, 5365–5370 (2014).
Xiang, F. et al. On-surface synthesis of chiral π-conjugate porphyrin tapes by substrate-regulated dehydrogenative coupling. J. Phys. Chem. C 123, 23007–23013 (2019).
Shubina, T. E. et al. Principle and mechanism of direct porphyrin metalation: joint experimental and theoretical investigation. J. Am. Chem. Soc. 129, 9476–9483 (2007).
Xiang, F. et al. Direct observation of copper-induced metalation of 5,15-diphenylporphyrin on Au(111) by scanning tunneling microscopy. Surf. Sci. 633, 46–52 (2015).
Müllegger, S. et al. Preserving charge and oxidation state of Au(III) ions in an agent-functionalized nanocrystal model system. ACS Nano 5, 6480–6486 (2011).
Eng, M. P., Ljungdahl, T., Andréasson, J., Mårtensson, J. & Albinsson, B. Triplet photophysics of gold(III) porphyrins. J. Phys. Chem. A 109, 1776–1784 (2005).
Cai, J. et al. Graphene nanoribbon heterojunctions. Nat. Nanotechnol. 9, 896–900 (2014).
Chen, Y.-C. et al. Molecular bandgap engineering of bottom-up synthesized graphene nanoribbon heterojunctions. Nat. Nanotechnol. 10, 156–160 (2015).
Power, P. P. Stable two-coordinate, open-shell (d1–d9) transition metal complexes. Chem. Rev. 112, 3482–3507 (2012).
Rubio-Verdú, C. et al. Orbital-selective spin excitation of a magnetic porphyrin. Commun. Phys. 1, 15 (2018).
Meng, X. et al. Controlling the spin states of FeTBrPP on Au(111). ACS Nano 17, 1268–1274 (2022).
Li, J. et al. Survival of spin state in magnetic porphyrins contacted by graphene nanoribbons. Sci. Adv. 4, eaaq0582 (2018).
Liu, B. et al. An iron-porphyrin complex with large easy-axis magnetic anisotropy on metal substrate. ACS Nano 11, 11402–11408 (2017).
Yu, M., Yang, S., Wu, C. & Marom, N. Machine learning the Hubbard U parameter in DFT+U using Bayesian optimization. npj Comput. Mater. 6, 180 (2020).
Himmetoglu, B., Floris, A., de Gironcoli, S. & Cococcioni, M. Hubbard-corrected DFT energy functionals: the LDA+U description of correlated systems. Int. J. Quantum Chem. 114, 14–49 (2014).
Ternes, M. Spin excitations and correlations in scanning tunneling spectroscopy. New J. Phys. 17, 063016 (2015).
Hahn, J. R. & Ho, W. Single molecule imaging and vibrational spectroscopy with a chemically modified tip of a scanning tunneling microscope. Phys. Rev. Lett. 87, 196102 (2001).
Giessibl, F. J. The qPlus sensor, a powerful core for the atomic force microscope. Rev. Sci. Instrum. 90, 011101 (2019).
Gross, L. et al. High-resolution molecular orbital imaging using a p-wave STM tip. Phys. Rev. Lett. 107, 086101 (2011).
Yakutovich, A. V. et al. AiiDAlab—an ecosystem for developing, executing, and sharing scientific workflows. Comput. Mater. Sci. 188, 110165 (2021).
Pizzi, G., Cepellotti, A., Sabatini, R., Marzari, N. & Kozinsky, B. AiiDA: automated interactive infrastructure and database for computational science. Comput. Mater. Sci. 111, 218–230 (2016).
Hutter, J., Iannuzzi, M., Schiffmann, F. & Vandevondele, J. Cp2k: atomistic simulations of condensed matter systems. Comput. Mol. Sci. 4, 15–25 (2014).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 154104 (2010).
Goedecker, S., Teter, M. & Hutter, J. Separable dual-space Gaussian pseudopotentials. Phys. Rev. B 54, 1703–1710 (1996).
VandeVondele, J. & Hutter, J. Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases. J. Chem. Phys. 127, 114105 (2007).
Hapala, P. et al. Mechanism of high-resolution STM/AFM imaging with functionalized tips. Phys. Rev. B 90, 085421 (2014).
Giannozzi, P. et al. Advanced capabilities for materials modelling with Quantum ESPRESSO. J. Phys. Condens. Matter 29, 465901 (2017).
Prandini, G., Marrazzo, A., Castelli, I. E., Mounet, N. & Marzari, N. Precision and efficiency in solid-state pseudopotential calculations. npj Comput. Mater. 4, 72 (2018).
Dudarev, S. L., Botton, G. A., Savrasov, S. Y., Humphreys, C. J. & Sutton, A. P. Electron-energy-loss spectra and the structural stability of nickel oxide: an LSDA+U study. Phys. Rev. B 57, 1505–1509 (1998).
Panchmatia, P. M., Sanyal, B. & Oppeneer, P. M. GGA+U modeling of structural, electronic, and magnetic properties of iron porphyrin-type molecules. Chem. Phys. 343, 47–60 (2008).
Ali, M. E., Sanyal, B. & Oppeneer, P. M. Electronic structure, spin-states, and spin-crossover reaction of heme-related Fe-porphyrins: a theoretical perspective. J. Phys. Chem. B 116, 5849–5859 (2012).
Tersoff, J. D. & Hamann, D. R. Theory of the scanning tunneling microscope. Phys. Rev. B 31, 805–813 (1985).
van Setten, M. J. et al. The PseudoDojo: training and grading a 85 element optimized norm-conserving pseudopotential table. Comput. Phys. Commun. 226, 39–54 (2018).
Perdew, J. P., Ernzerhof, M. & Burke, K. Rationale for mixing exact exchange with density functional approximations. J. Chem. Phys. 105, 9982–9985 (1996).
Adamo, C. & Barone, V. Toward reliable density functional methods without adjustable parameters: the PBE0 model. J. Chem. Phys. 110, 6158–6170 (1999).
Lin, L. Adaptively compressed exchange operator. J. Chem. Theory Comput. 12, 2242–2249 (2016).
Xiang, F. et al. Zigzag graphene nanoribbons with periodic porphyrin edge extensions. Materials Cloud https://doi.org/10.24435/materialscloud:bw-ph (2025).
Eroshin, A. V., Koptyaev, A. I., Otlyotov, A. A., Minenkov, Y. & Zhabanov, Y. A. Iron(II) complexes with porphyrin and tetrabenzoporphyrin: CASSCF/MCQDPT2 study of the electronic structures and UV–Vis spectra by sTD-DFT. Int. J. Mol. Sci. 24, 7070 (2023).
Antalík, A. et al. Ground state of the Fe(II)-porphyrin model system corresponds to quintet: a DFT and DMRG-based tailored CC study. Phys. Chem. Chem. Phys. 22, 17033–17037 (2020).
Acknowledgements
This research was supported by the Swiss National Science Foundation under grant no. 212875, the NCCR MARVEL, a National Centre of Competence in Research funded by the Swiss National Science Foundation (grant no. 205602), the Werner Siemens Foundation (CarboQuant), the Max Planck Society, ERC grant nos. NANOGRAPH and 2DMATER, EU Project GENIUS, the EC under Graphene Flagship (grant no. CNECT-ICT-604391), the Hundred Talented Program of the Chinese Academy of Science, Natural Science Foundation of Zhejiang Province (grant no. LZ24B020006), Natural Science Foundation of China (grant no. 22405284) and start-up funding at the Chinese University of Hong Kong, Shenzhen (grant no. UDF01002468). C.A.P. acknowledges the Swiss Supercomputing Centre (CSCS) for computing resources (project s1267, lp83). F.X. thanks the Deutsche Forschungsgemeinschaft (DFG) for a Walter-Benjamin Fellowship (project grant no. 452269487) and the SNSF Swiss Postdoctoral Fellowship (grant no. 210093). Y.G. and Z.Q. acknowledge Humboldt Research Fellowships from the Alexander von Humboldt Stiftung. K.M. acknowledges a fellowship from Gutenberg Research College, Johannes Gutenberg University Mainz.
Author information
Authors and Affiliations
Contributions
A.K., R.F., P.R. and K.M. conceived of this project. Y.G. synthesized the precursors under the supervision of Z.Q. and K.M. F.X., A.K. and N.B. performed the scanning probe microscopy measurements. F.X. analysed the scanning probe microscopy data under the supervision of P.R. and R.F. F.X., A.O.-G., O.G. and C.A.P. performed the DFT calculations and theoretical analyses. F.X. and R.F. wrote the original draft. All the co-authors participated in the scientific discussions and in reviewing and editing the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Ping Yu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Differential conductance dI/dV measurements for ZnPor-3ZGNRs segments of increasing length.
(a) STM images of ZnPor-3ZGNR dimer (NZnPor = 2), trimer (NZnPor = 3), tetramer (NZnPor = 4), pentamer (NZnPor = 5) and hexamer (NZnPor = 6). The length of ZnPor-3ZGNR is defined by the number N of ZnPor units in the hybrid ribbon. The color dots in each STM image refer to the tip positions where the dI/dV spectra were taken. (b) dI/dV spectra taken from dimer to hexamer. The color of each spectrum corresponds to the color of the dots shown in (a). The arrows highlight the frontier states that define the bandgap. Both the HOMO (developing into the valence band, VB) and the LUMO (developing into the conduction band, CB) move towards the Fermi level as the length of the ZnPor-3ZGNR increases. From the pentamer onwards, the HOMO / VB resonance disappears from the spectrum due to its dispersive character. The LUMO / CB position saturates at 0.2 eV in the hexamer. (c) Comparison of experimental (Exp) and DFT calculated (DFT_GP refers to gas phase calculations, DFT_AuSub refers to calculations with Au(111) substrate) bandgaps of ZnPor-3ZGNR dimer to hexamer. Although the calculated band gaps are underestimated by DFT, the trend of decreasing band gap as ribbon length increases is well reproduced. (a) U = −1.5 V, I = 100 pA for dimer to hexamer.
Extended Data Fig. 2 Nc-AFM images and differential conductance dI/dV maps of ZnPor-3ZGNR with different lengths.
Nc-AFM image (first column) and experimental CH dI/dV maps (second to fifth column) of (a) dimer, (b) trimer, (c) tetramer, and (d) hexamer acquired with a CO-functionalized tip. Scanning parameters: (a)-(d) nc-AFM images: U = 0.01 V, I = 250 pA before switching off the feedback. dI/dV maps: I = 500 pA, bias voltages are shown on top of each map.
Extended Data Fig. 3 DFT calculations for Zn-porphine (ZnPor) in gas phase, performed at the PBE level of theory.
(a) Geometry-optimized ZnPor in gas phase. Black, white, blue and grey balls represent carbon, hydrogen, nitrogen and zinc, respectively. (b) Calculated molecular orbitals, evaluated in a plane 3 Å above the molecule. The HOMO and HOMO-1 are close in energy. The LUMO consists of two degenerate orbitals.
Extended Data Fig. 4 Spin-polarized DFT calculations.
The calculated band structure, density of states (DOS) (broadening: 0.05 eV) and LDOS maps of (a) pristine 3ZGNR with primitive unit cell, (b) pristine 3ZGNR in supercell, and (c) strained 3ZGNR. The supercell of the pristine 3ZGNR considered in (b) has been chosen to contain the same number of units as the unit cell of the strained 3ZGNR in (c). In order to maintain the strain pattern consistent with that of the ZnPor-3ZGNR, the strained 3ZGNR model in (c) was built by removing the Por units from the geometry optimized ZnPor-3ZGNR. The unsaturated carbon atoms were then passivated with hydrogen atoms. During geometry optimization of the resulting structure, the backbone of the 3ZGNR was fixed, allowing only the hydrogen atoms to relax. Simulated LDOS maps and spin density maps are shown in the third column. These were evaluated in a plane 3 Å above the ribbon.
Extended Data Fig. 5 Projection of the states of a 12-unit long ZnPor-3ZGNR onto the frontier orbitals of a ZnPor molecule.
Left: Calculated band structure from an infinite ZnPor-3ZGNR using QE code. The corresponding DOS (yellow) is shown on the right. The DOS of an infinite ZnPor-3ZGNR is rescaled to have better visualization. Right: The projected DOS (pDOS) of ZnPor-3ZGNR dodecamer onto HOMO-1 (blue), HOMO (dark blue), LUMO (red) of ZnPor and pDOS of ZnPor-3ZGNR dodecamer (grey). The ZnPor-3ZGNR dodecamer was calculated in periodic boundary condition at the Γ point using the CP2K code. The dodecamer calculation is equivalent to a unit-cell calculation with 12 k-points. Comparing to the calculated band structure and DOS from an infinite hybrid ribbon, ZnPor units show major contribution to CB + 1 and VB-1 in the hybrid ribbon. The LUMO and HOMO of ZnPor become highly dispersive after fusing to 3ZGNR, implying the formation of large π-extension across ZnPor units and 3ZGNR in the hybrid system.
Extended Data Fig. 6 On-surface synthesis of 2HPor-3ZGNRs.
(a) On-surface synthetic route to 2HPor-3ZGNR using 2HPor-DBA precursor. (b) STM image of 2HPor-DBA on Au(111) after heating to 415 K for 30 min. The precursors have undergone debrominative aryl-aryl coupling and formed 2HPor-3ZGNR polymers on the surface. (c) 2HPor-3ZGNR is obtained through cyclodehydrogenative coupling by heating the sample to 620 K for 30 min. High-resolution STM images of 2HPor-3ZGNR with AuPor units, scanned at (d) 0.1 V and (e) −1.13 V. (f) CH nc-AFM image of the 2HPor-3ZGNR with AuPor units shown in (d) and (e). (g) Chemical structure of the ribbon segment imaged in (f). Black, white, blue and red balls represent carbon, hydrogen, nitrogen and metalated gold atoms, respectively. Scanning parameters: (b) U = 0.1 V, I = 100 pA. (c) U = −1.5 V, I = 100 pA. (d) U = 0.1 V, I = 100 pA. (e) U = −1.13 V, I = 100 pA. (f) The tip position is defined on top of the 3ZGNR at U = 0.005 V, I = 150 pA before turning off the feedback.
Extended Data Fig. 7 DFT calculated band structure and local density of states of MPor-3ZGNRs (M = 2H, Zn, Au) in gas phase.
Chemical structure of (a) 2HZnPor-3ZGNR, (d) ZnPor-3ZGNR, and (g) AuPor-3ZGNR. The calculated band structure of (b) 2HZnPor-3ZGNR, (e) ZnPor-3ZGNR, and (h) AuPor-3ZGNR at PBE level of theory. The valence bands and conduction bands for each system are marked as VB-1, VB, CB and CB + 1, respectively. In AuPor-3ZGNR, due to the electron transfer from the Au center to the Por-3ZGNR ligand, the previous CB of Por-3ZGNR is fully occupied and moves below the Fermi level. Thus the VB-1, VB, CB and CB + 1 for Por-3ZGNR are marked as B-1, B-2, B-3 and B-4. The calculated wave function squared frontier bands at the Γ point for (c) 2HZnPor-3ZGNR, (f) ZnPor-3ZGNR and (i) AuPor-3ZGNR, evaluated in a plane 3 Å above the ribbon (FWHM broadening = 0.1 eV).
Extended Data Fig. 8 Spin-polarized DFT calculations of density of states of 13-unit long 2HPor-3ZGNR with one metalated AuPor unit on Au(111).
(a) Geometry optimized model of the 2HPor-3ZGNR with one AuPor unit on Au(111). Black, white, blue, red, and yellow balls represent carbon, hydrogen, nitrogen, the metalated Au, and surface Au atoms, respectively. (b) Calculated DOS (broadening: 0.05 eV) of the 2HPor-3ZGNR with one AuPor (grey shaded area) on Au(111). The projected orbitals on to the Au center in AuPor (green shaded area), the carbon backbone of the AuPor unit (marked by arrow in (a), blue line in DOS map) and the 2HPor unit (marked by arrow in (a), pink line in DOS map) are shown in the map. The Au state is located in the unoccupied region, indicating an electron donation to the ligand. (c) Simulated LDOS maps for the energies indicated by dashed lines in (b). The LDOS maps at −0.05 eV, 0.08 eV, and 0.2 eV were simulated with FWHM broadening of 0.02 eV, the rest using FWHM broadening of 0.2 eV (LDOS evaluated in a plane 3 Å above the ribbon). The same LDOS maps are also shown in Fig. 4(e).
Extended Data Fig. 9 On-surface synthesis of FePor-3ZGNR.
(a) On-surface synthetic route to FePor-3ZGNR using FePorCl-DBA precursors. While there is a Cl atom attached to the Fe center of the as-synthesized FePor-DBA precursor, degassing of the compound under UHV conditions detaches Cl before actual precursor sublimation. (b) Overview STM image of the sample after annealing to 570 K for 15 min. FePor-3ZGNR is fully planarized. (c) High-resolution STM images of FePor-3ZGNRs of different lengths, specified by the number NFePor of precursor units. (d) dI/dV spectra taken from dimer (NFePor = 2) to hexamer (NFePor = 6), at locations indicated by the colored dots in (c). The arrows indicate the position of detected resonances. As judged from dI/dV maps taken at corresponding energies (see Supplementary Fig. 17), resonances 1, 2, 5 and 6 exhibit the typical LDOS features of the VB-1, VB, CB and CB + 1, respectively, of the Por-3ZGNR backbone, as also observed for the ZnPor-3ZGNR. Resonance 3 and 4 are specific to the FePor-3ZGNR, that is they are not observed in ZnPor-3ZGNR. Scanning parameters: (b) U = −1.5 V, I = 100 pA. (c) U = −1.5 V, I = 300 pA.
Extended Data Fig. 10 DFT calculation of Fe in FePor-3ZGNR with different spin configurations and electron occupation in Fe.
(a) Spin configuration considered for the Fe(II) in the porphine core. (b) Using the FePor-3ZGNR structure optimized within PBE level of theory, we use single point calculations with DFT + U (U = 4.4 eV) to determine the spin configuration per Fe atom. The most stable spin configuration is S = 1 per Fe atom. This result is in agreement with previously reported DFT calculations64,65. (c) Scheme of electron occupation in Fe 3 d orbitals with DFT + U (U = 4.4 eV). The two Fe atoms in the FePor-3ZGNR unit cell are specified as Fe1 and Fe2 respectively. \({d}_{{z}^{2}}\) and \({d}_{{yz}}\) are singly occupied, giving the total spin of S = 1 in each FePor unit.
Supplementary information
Supplementary Information
Synthetic procedures, Supplementary Figs. 1–31, Spectroscopic Data Figs. 32–51, Tables 1–6 and refs. 1–9.
Supplementary Data 1
Crystallographic data for 2HPor–DBA; CCDC reference 2281373.
Supplementary Data 2
Crystallographic data for ZnPor–DBA; CCDC reference 2281312.
Supplementary Data 3
Crystallographic data for FePorOMe–DBA; CCDC reference 2391152.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiang, F., Gu, Y., Kinikar, A. et al. Zigzag graphene nanoribbons with periodic porphyrin edge extensions. Nat. Chem. 17, 1356–1363 (2025). https://doi.org/10.1038/s41557-025-01887-9
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01887-9
This article is cited by
-
Merging d- and π-electron magnetism
Nature Chemistry (2025)