Abstract
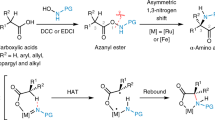
Incorporating unnatural amino acids, such as hindered N-methylated or α,α-disubstituted amino acid(s), into peptides can improve their properties for application in the pharmaceutical and biomedical fields. However, the current solid-phase peptide synthesis (SPPS) faces sluggish reaction rates and low yields when incorporating sterically hindered amino acids, owing to the poor kinetics of the two-phase acyl-transfer process from solution to solid. Here we introduce an immobilized ribosome-mimicking molecular reactor to facilitate on-resin proximity-induced intra(inter)-reactor acyl transfers. This strategy bypasses the two-phase acyl-transfer mechanism in conventional SPPS and boosts coupling efficiency in the solid-phase synthesis of N-methylated and/or α,α-disubstituted amino acid(s)-containing sterically hindered peptides, including cyclosporin A and alamethicin F analogues. The ribosome-mimicking molecular reactor SPPS can be integrated into existing SPPS platforms using commercially available resins and reagents, and displays high compatibility with standard synthesizers, enabling the automated synthesis of pharmaceutically important, sterically hindered difficult peptides.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data are available in the main text and the Supplementary Information. Source data are provided with this paper.
References
Jensen, K. J., Shelton, P. T. & Pedersen, S. L. (eds.) Peptide Synthesis and Applications 2nd edn (Humana, 2013).
Vinogradov, A. A., Yin, Y. & Suga, H. Macrocyclic peptides as drug candidates: recent progress and remaining challenges. J. Am. Chem. Soc. 141, 4167–4181 (2019).
Muttenthaler, M., King, G. F., Adams, D. J. & Alewood, P. F. Trends in peptide drug discovery. Nat. Rev. Drug Discov. 20, 309–325 (2021).
Henninot, A., Collins, J. C. & Nuss, J. M. The current state of peptide drug discovery: back to the future? J. Med. Chem. 61, 1382–1414 (2018).
Chatterjee, J., Gilon, C., Hoffman, A. & Kessler, H. N-Methylation of peptides: a new perspective in medicinal chemistry. Acc. Chem. Res. 41, 1331–1342 (2008).
Hamman, J. H., Enslin, G. M. & Kotzé, A. F. Oral delivery of peptide drugs: barriers and developments. BioDrugs 19, 165–177 (2005).
Chatterjee, J., Rechenmacher, F. & Kessler, H. N-Methylation of peptides and proteins: an important element for modulating biological functions. Angew. Chem. Int. Ed. 52, 254–269 (2013).
Biron, E. et al. Improving oral bioavailability of peptides by multiple N-methylation: somatostatin analogues. Angew. Chem. Int. Ed. 47, 2595–2599 (2008).
Driggers, E. M., Hale, S. P., Lee, J. & Terrett, N. K. The exploration of macrocycles for drug discovery—an underexploited structural class. Nat. Rev. Drug Discov. 7, 608–624 (2008).
Toniolo, C., Formaggio, F., Kaptein, B. & Broxterman, Q. B. You are sitting on a gold mine! Synlett 9, 1295–1310 (2006).
Merrifield, R. B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 85, 2149–2154 (1963).
Merrifield, R. B. Automated synthesis of peptides. Science 150, 178 (1965).
Naoum, J. N., Alshanski, I., Mayer, G., Strauss, P. & Hurevich, M. Stirring peptide synthesis to a new level of efficiency. Org. Process Res. Dev. 26, 129–136 (2022).
Alshanski, I. et al. Enhancing the efficiency of the solid phase peptide synthesis (SPPS) process by high shear mixing. Org.Process Res. Dev. 22, 1318–1322 (2018).
Naoum, J. N. et al. Diffusion-enhanced amide bond formation on a solid support. Org. Process Res. Dev. 23, 2733–2739 (2019).
Falb, E., Yechezkel, T., Salitra, Y. & Gilon, C. In situ generation of Fmoc-amino acid chlorides using bis-(trichloromethyl) carbonate and its utilization for difficult couplings in solid-phase peptide synthesis. J. Pept. Res. 53, 507–517 (1999).
Frérot, E., Coste, J., Pantaloni, A., Dufour, M.-N. & Jouin, P. PyBOP and PyBroP: Two reagents for the difficult coupling of the α,α-dialkyl amino acid, Aib. Tetrahedron 47, 259–270 (1991).
Otake, Y. et al. N-Methylated peptide synthesis via generation of an acyl N-methylimidazolium cation accelerated by a Broensted acid. Angew. Chem. Int. Ed. 59, 12925–12930 (2020).
Wang, X., Li, J. & Hayashi, Y. Highly sterically hindered peptide bond formation between α,α-disubstituted α-amino acids and N-alkyl cysteines using α,α-disubstituted α-amidonitrile. J. Am. Chem. Soc. 144, 10145–10150 (2022).
Crick, F. Central dogma of molecular biology. Nature 227, 561–563 (1970).
Steitz, T. A. A structural understanding of the dynamic ribosome machine. Nat. Rev. Mol. Cell Biol. 9, 242–253 (2008).
Mäde, V., Els-Heindl, S. & Beck-Sickinger, A. G. Automated solid-phase peptide synthesis to obtain therapeutic peptides. Beilstein J. Org. Chem. 10, 1197–1212 (2014).
König, W. & Geiger, R. Eine neue methode zur synthese von peptiden: aktivierung der carboxylgruppe mit dicyclohexylcarbodiimid unter zusatz von 1-hydroxy-benzotriazolen. Chem. Ber. 103, 788–798 (1970).
Subirós-Funosas, R., Prohens, R., Barbas, R., El-Faham, A. & Albericio, F. Oxyma: an efficient additive for peptide synthesis to replace the benzotriazole-based HOBt and HOAt with a lower risk of explosion[1]. Chem. Eur. J. 15, 9394–9403 (2009).
Bhattacharya, S. & Kumar, V. P. Ester cleavage properties of synthetic hydroxybenzotriazoles in cationic monovalent and gemini surfactant micelles. Langmuir 21, 71–78 (2005).
He, X. & Jabbari, E. Solid-phase synthesis of reactive peptide crosslinker by selective deprotection. Protein Pept. Lett. 13, 715–718 (2006).
Hines, D. A. & Kamat, P. V. Quantum dot surface chemistry: ligand effects and electron transfer reactions. J. Phys. Chem. C 117, 14418–14426 (2013).
Greenwald, R. B. PEG drugs: an overview. J. Control. Release 74, 159–171 (2001).
Suzuki, Y. et al. Inflammation and angiotensin II. Int. J. Biochem. Cell Biol. 35, 881–900 (2003).
Kumar, P., Paterson, N. G., Clayden, J. & Woolfson, D. N. De novo design of discrete, stable 310-helix peptide assemblies. Nature 607, 387–392 (2022).
Yang, Y. & Hansen, L. Optimized Fmoc-removal strategy to suppress the traceless and conventional diketopiperazine formation in solid-phase peptide synthesis. ACS Omega 7, 12015–12020 (2022).
Subirós-Funosas, R., El-Faham, A. & Albericio, F. PyOxP and PyOxB: the Oxyma-based novel family of phosphonium salts. Org. Biomol. Chem. 8, 3665–3673 (2010).
Nzama, H., Manne, S. R., Akintayo, D. C., de la Torre, B. G. & Albericio, F. Ethylthio-1H-tetrazole (ETT) as coupling additive for the solid-phase synthesis (SPS) of hindered amino acid-containing peptides. Tetrahedron Lett. 142, 155108 (2024).
Ahlbach, C. L. et al. Beyond cyclosporine A: conformation-dependent passive membrane permeabilities of cyclic peptide natural products. Future Med. Chem. 7, 2121–2130 (2015).
Ruegger, A. et al. Cyclosporin A, ein immunsuppressiv wirksamer peptidm-etabolit aus trichoderma polysporum (link ex pers.) rifai. Helv. Chim. Acta 59, 1075–1092 (1976).
Attur, M. G. et al. Differential anti-inflammatory effects of immunosuppressive drugs: cyclosporin, rapamycin and FK-506 on inducible nitric oxide synthase, nitric oxide, cyclooxygenase-2 and PGE 2 production. Inflammation Res. 49, 20–26 (2000).
Corbett, K. M., Ford, L., Warren, D. B., Pouton, C. W. & Chalmers, D. K. Cyclosporin structure and permeability: from A to Z and beyond. J. Med. Chem. 64, 13131–13151 (2021).
Li, P. & Xu, J. C. Total synthesis of cyclosporin O both in solution and in the solid phase using novel thiazolium-, immonium-, and pyridinium-type coupling reagents: BEMT, BDMP, and BEP1. J. Org. Chem. 65, 2951–2958 (2000).
Angell, Y. M., Thomas, T. L., Flentke, G. R. & Rich, D. H. Solid-phase synthesis of cyclosporin peptides. J. Am. Chem. Soc. 117, 7279–7280 (1995).
Sharma, A. et al. N-methylation in amino acids and peptides: scope and limitations. Biopolymers 109, 23110–23124 (2018).
Urban, J. A. N., Vaisar, T. O. M., Shen, R. & Lee, M. S. Lability of N-alkylated peptides towards TFA cleavage. Int. J. Pept. Protein Res. 47, 182–189 (1996).
Sheppard, R. C. & Williams, B. J. Acid-labile resin linkage agents for use in solid phase peptide synthesis. Int. J. Pept. Protein Res. 20, 451–454 (1982).
Zieleniewski, F., Woolfson, D. N. & Clayden, J. Automated solid-phase concatenation of Aib residues to form long, water-soluble, helical peptides. Chem. Commun. 56, 12049–12052 (2020).
Pike, S. J., Raftery, J., Webb, S. J. & Clayden, J. Conformational analysis of helical aminoisobutyric acid (Aib) oligomers bearing C-terminal ester Schellman motifs. Org. Biomol. Chem. 12, 4124–4131 (2014).
Zhang, Y., Vanderghinste, J., Wang, J. & Das, S. Challenges and recent advancements in the synthesis of α,α-disubstituted α-amino acids. Nat. Commun. 15, 1474 (2024).
Whitmore, L. & Wallace, B. A. Analysis of peptaibol sequence composition: implications for in vivo synthesis and channel formation. Eur. Biophys. J. 33, 233–237 (2004).
Chugh, J. K. & Wallace, B. A. Peptaibols: models for ion channels. Biochem. Soc. Trans. 29, 565–570 (2001).
Casagrande, N. et al. Analogs of a natural peptaibol exert anticancer activity in both cisplatin-and doxorubicin-resistant cells and in multicellular tumor spheroids. Int. J. Mol. Sci. 22, 8362 (2021).
Shi, M. et al. Antimicrobial peptaibols, novel suppressors of tumor cells, targeted calcium-mediated apoptosis and autophagy in human hepatocellular carcinoma cells. Mol. Cancer 9, 1–16 (2010).
Whitmore, L. & Wallace, B. A. The Peptaibol Database: a database for sequences and structures of naturally occurring peptaibols. Nucleic Acids Res. 32, D593–D594 (2004).
Nagaraj, R. & Balaram, P. Alamethicin, a transmembrane channel. Acc. Chem. Res. 14, 356–362 (1981).
Siow, A., Hung, K. Y., Harris, P. W. R. & Brimble, M. A. Solid-phase synthesis of the peptaibol alamethicin U-22324 by using a double-linker strategy. Eur. J. Org. Chem. 2017, 350–354 (2017).
Hjørringgaard, C. U., Pedersen, J. M., Vosegaard, T., Nielsen, N. C. & Skrydstrup, T. An automatic solid-phase synthesis of peptaibols. J. Org. Chem. 74, 1329–1332 (2009).
Ben Haj Salah, K. & Inguimbert, N. Efficient microwave-assisted one shot synthesis of peptaibols using inexpensive coupling reagents. Org. Lett. 16, 1783–1785 (2014).
Acknowledgements
The authors thank the National Natural Science Foundation of China (grant no. 22450003) and Shenzhen Science and Technology Program (grant no. KQTD20190929174023858) for financial support, and Y. Du for assistance in mass spectroscopy analysis.
Author information
Authors and Affiliations
Contributions
S.W. developed and optimized the RMMRs, completed most of the experiments and data collection. X.Z. and X.Y. participated in the partial synthetic experiments, NMR experiments and spectra analyses. S.W., F.L. and Z.-J.Y. wrote the manuscript, with inputs from all authors. F.L. and Z.-J.Y. conceptualized the project and participated in the design and coordination of the experiments.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, experimental procedures, compound characterizations, LC-MS analysis reports and copies of 1H and 13C NMR spectra.
Supplementary Data
Packed raw NMR data
Source data
Source Data Fig. 3
Source data for HPLC analysis in Fig. 3c,d,e.
Source Data Fig. 4
Source data for HPLC analysis in Fig. 4b.
Source Data Fig. 5
Source data for HPLC analysis in Fig. 5.
Source Data Fig. 6
Source data for HPLC analysis in Fig. 6ab.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wei, S., Zhang, X., Yang, X. et al. Immobilized acyl-transfer molecular reactors enable the solid-phase synthesis of sterically hindered peptides. Nat. Chem. 17, 1596–1606 (2025). https://doi.org/10.1038/s41557-025-01896-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01896-8