Abstract
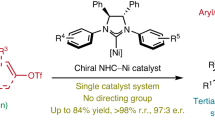
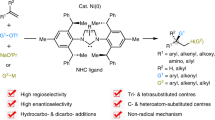
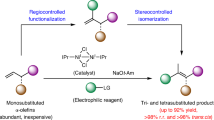
Precision in site-selective functionalization of molecules bearing multiple potentially reactive positions is a longstanding challenge in organic synthesis. Although migratory difunctionalization offers a powerful strategy for programmed functional group installation along carbon chains, its implementation with multisubstituted alkenes has been impeded by the increased complexity of site-selectivity. Here we report a head–tail carboboration of multisubstituted alkenes with exceptional site-selectivity, enabled by ligand steric exclusion in a nickel-catalysed chain-walking system. This catalytic system enables selective migratory transformations of tertiary alkyl–metal intermediates across a range of thermodynamically and kinetically accessible reaction site combinations. The importance of this study is evident in not only its capacity to efficiently couple structurally diverse carbon electrophiles with complex substrates, including natural terpenes, but also its contribution to streamlining the synthesis of natural products and materials.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available in the article and its Supplementary Information. Crystallographic data for the structures reported in this article have been deposited at the Cambridge Crystallographic Data Centre, under deposition numbers CCDC 2376317 (52), CCDC 2376318 (54-1) and CCDC 2313837 (56-1). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/.
References
Trost, B. M. Selectivity: a key to synthetic efficiency. Science 219, 245–250 (1983).
Clayden, J., Greeves, N. & Warren, S. Organic Chemistry (Oxford Univ. Press, 2012).
Zhu, J., Wang, Q. & Wang, M. Multicomponent Reactions in Organic Synthesis (Wiley, 2014).
Davies, H. M. L. & Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 3, 347–360 (2019).
He, J., Wasa, M., Chan, K. S. L., Shao, Q. & Yu, J.-Q. Palladium-catalyzed transformations of alkyl C–H bonds. Chem. Rev. 117, 8754–8786 (2017).
McDonald, R. I., Liu, G. & Stahl, S. S. Palladium(II)-catalyzed alkene functionalization via nucleopalladation: stereochemical pathways and enantioselective catalytic applications. Chem. Rev. 111, 2981–3019 (2011).
Whyte, A., Torelli, A., Mirabi, B., Zhang, A. & Lautens, M. Copper-catalyzed borylative difunctionalization of π-systems. ACS Catal. 10, 11578–11622 (2020).
Yin, G., Mu, X. & Liu, G. Palladium(II)-catalyzed oxidative difunctionalization of alkenes: bond forming at a high-valent palladium center. Acc. Chem. Res. 49, 2413–2423 (2016).
Wu, D. et al. Overcoming limitations in non-activated alkene cross-coupling with nickel catalysis and anionic ligands. Nat. Catal. 7, 1154–1164 (2024).
Wickham, L. M. & Giri, R. Transition metal (Ni, Cu, Pd)-catalyzed alkene dicarbofunctionalization reactions. Acc. Chem. Res. 54, 3415–3437 (2021).
Li, Y. Y. & Yin, G. Y. Nickel chain-walking catalysis: a journey to migratory carboboration of alkenes. Acc. Chem. Res. 56, 3246–3259 (2023).
Sommer, H., Juliá-Hernández, F., Martin, R. & Marek, I. Walking metals for remote functionalization. ACS Cent. Sci. 4, 153–165 (2018).
Vasseur, A., Bruffaerts, J. & Marek, I. Remote functionalization through alkene isomerization. Nat. Chem. 8, 209–219 (2016).
Rodrigalvarez, J., Haut, F.-L. & Martin, R. Regiodivergent sp3 C–H functionalization via Ni-catalyzed chain-walking reactions. JACS Au 3, 3270–3282 (2023).
Li, Y., Wu, D., Cheng, H.-G. & Yin, G. Difunctionalization of alkenes involving metal migration. Angew. Chem. Int. Ed. 59, 7990–8003 (2020).
Ding, C., Ren, Y., Sun, C., Long, J. & Yin, G. Regio- and stereoselective alkylboration of endocyclic olefins enabled by nickel catalysis. J. Am. Chem. Soc. 143, 20027–20034 (2021).
Pang, H., Wu, D., Cong, H. & Yin, G. Stereoselective palladium-catalyzed 1,3-arylboration of unconjugated dienes for expedient synthesis of 1,3-disubstituted cyclohexanes. ACS Catal. 9, 8555–8560 (2019).
Clardy, J. & Walsh, C. Lessons from natural molecules. Nature 432, 829–837 (2004).
Sharifi-Rad, J. et al. Biological activities of essential oils: from plant chemoecology to traditional healing systems. Molecules 22, 70 (2017).
Lam, Y. A., Lawson, T. G., Velayutham, M., Zweier, J. L. & Pickart, C. M. A proteasomal ATPase subunit recognizes the polyubiquitin degradation signal. Nature 416, 763–767 (2002).
Rahighi, S. et al. Specific recognition of linear ubiquitin chains by NEMO is important for NF-κB activation. Cell 136, 1098–1109 (2009).
Beal, R. E., Toscano-Cantaffa, D., Young, P., Rechsteiner, M. & Pickart, C. M. The hydrophobic effect contributes to polyubiquitin chain recognition. Biochemistry 37, 2925–2934 (1998).
Qian, C. & Zhou, M. M. SET domain protein lysine methyltransferases: structure, specificity and catalysis. Cell. Mol. Life Sci. 63, 2755–2763 (2006).
Wu, H. et al. Structural biology of human H3K9 methyltransferases. PLoS ONE 5, e8570 (2010).
Luo, M. Chemical and biochemical perspectives of protein lysine methylation. Chem. Rev. 118, 6656–6705 (2018).
Bhat, K. P., Kaniskan, H. Ü., Jin, J. & Gozani, O. Epigenetics and beyond: targeting writers of protein lysine methylation to treat disease. Nat. Rev. Drug. Discov. 20, 265–286 (2021).
Botuyan, M. V. et al. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell 127, 1361–1373 (2006).
Braude, E. A., Coles, J. A., Evans, E. A. & Timmons, C. J. Steric effects in anionotropic rearrangements. Nature 177, 1167–1169 (1956).
Ramadoss, B., Jin, Y., Asako, S. & Ilies, L. Remote steric control for undirected meta-selective C–H activation of arenes. Science 375, 658–663 (2022).
Wu, D. et al. Alkene 1,1-difunctionalizations via organometallic-radical relay. Nat. Catal. 6, 1030–1041 (2023).
Li, Y. et al. Modular access to substituted cyclohexanes with kinetic stereocontrol. Science 376, 749–753 (2022).
Wang, W., Ding, C. & Yin, G. Catalyst-controlled enantioselective 1,1-arylboration of unactivated olefins. Nat. Catal. 3, 951–958 (2020).
Sakai, H. A., Liu, W., Le, C. C. & MacMillan, D. W. C. Cross-electrophile coupling of unactivated alkyl chlorides. J. Am. Chem. Soc. 142, 11691–11697 (2020).
Logan, K. M., Sardini, S. R., White, S. D. & Brown, M. K. Nickel-catalyzed stereoselective arylboration of unactivated alkenes. J. Am. Chem. Soc. 140, 159–162 (2018).
Joung, S., Bergmann, A. M. & Brown, M. K. Ni-catalyzed 1,2-benzylboration of 1,2-disubstituted unactivated alkenes. Chem. Sci. 10, 10944–10947 (2019).
Jansen, D. J. & Shenvi, R. A. Synthesis of medicinally relevant terpenes: reducing the cost and time of drug discovery. Future Med. Chem. 6, 1127–1146 (2014).
Wang, S. et al. Cobalt-catalysed allylic fluoroalkylation of terpenes. Nat. Synth. 2, 1202–1210 (2023).
Nairoukh, Z., Cormier, M. & Marek, I. Merging C–H and C–C bond cleavage in organic synthesis. Nat. Rev. Chem. 1, 0035 (2017).
Di Martino, R. M. C., Maxwell, B. D. & Pirali, T. Deuterium in drug discovery: progress, opportunities and challenges. Nat. Rev. Drug. Discov. 22, 562–584 (2023).
Li, Z. et al. Nickel-catalyzed regio- and enantioselective borylative coupling of terminal alkenes with alkyl halides enabled by an anionic bisoxazoline ligand. J. Am. Chem. Soc. 145, 13603–13614 (2023).
Sun, C., Ding, C., Yu, Y., Li, Y. & Yin, G. Ligand-modulated regiodivergent alkenylboration of allylarenes: reaction development and mechanistic study. Fundam. Res. https://doi.org/10.1016/j.fmre.2023.03.016 (2023).
Hansch, C., Leo, A. & Taft, R. W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 91, 165–195 (1991).
Falivene, L. et al. Towards the online computer-aided design of catalytic pockets. Nat. Chem. 11, 872–879 (2019).
Falivene, L. et al. SambVca 2. A web tool for analyzing catalytic pockets with topographic steric maps. Organometallics 35, 2286–2293 (2016).
Escayola, S., Bahri-Laleh, N. & Poater, A. %VBur index and steric maps: from predictive catalysis to machine learning. Chem. Soc. Rev. 53, 853–882 (2024).
Crabtree, R. H. The Organometallic Chemistry of the Transition Metals (Wiley, 2009).
Li, X. et al. Regio- and enantioselective remote dioxygenation of internal alkenes. Nat. Chem. 15, 862–871 (2023).
Matteson, D. S. Stereodirected Synthesis with Organoboranes (Springer Science & Business Media, 2012).
Sandford, C. & Aggarwal, V. K. Stereospecific functionalizations and transformations of secondary and tertiary boronic esters. Chem. Commun. 53, 5481–5494 (2017).
Li, C. et al. Non-fullerene acceptors with branched side chains and improved molecular packing to exceed 18% efficiency in organic solar cells. Nat. Energy 6, 605–613 (2021).
Gao, Y. et al. All-small-molecule organic solar cells with 18.1% efficiency and enhanced stability enabled by improving light harvesting and nanoscale microstructure. Joule 7, 2845–2858 (2023).
Chen, C. L. et al. Tetrahedral liquid-crystalline networks: an A15-like Frank–Kasper phase based on rod-packing. Angew. Chem. Int. Ed. 61, e202203447 (2022).
Schumm, J. S., Pearson, D. L. & Tour, J. M. Iterative divergent/convergent approach to linear conjugated oligomers by successive doubling of the molecular length: a rapid route to a 128 Å-long potential molecular wire. Angew. Chem. Int. Ed. 33, 1360–1363 (2003).
Yang, Z. et al. Reptilian chemistry: characterization of a family of dianeackerone-related steroidal esters from a crocodile secretion. Proc. Natl Acad. Sci. USA 96, 12251–12256 (1999).
Krückert, K., Flachsbarth, B., Schulz, S., Hentschel, U. & Weldon, P. J. Ethyl-branched aldehydes, ketones, and diketones from caimans (Caiman and Paleosuchus; Crocodylia, Reptilia). J. Nat. Prod. 69, 863–870 (2006).
Yang, Z., Attygalle, A. B. & Meinwald, J. Reptilian chemistry: enantioselective syntheses of novel components from a crocodile exocrine secretion. Synthesis 2000, 1936–1943 (2000).
Acknowledgements
National Natural Science Foundation of China (grant nos. 22122107 to G.Y. and 22401220 to Yangyang Li), Guangdong Basic and Applied Basic Research Foundation (grant no. 2024A1515011689 to G.Y.), Fundamental Research Funds for the Central Universities (grant no. 413100070 to Yangyang Li) and Scientific Research Innovation Capability Support Project for Young Faculty (ZYGXQNJSKYCXNLZCXM-H17 to G.Y.) are acknowledged for financial support. We thank J. Min from Wuhan University for providing the intermediate compounds essential for synthesizing organic solar cells. We also thank the Core Facility of Wuhan University for their assistance with X-ray crystallographic analysis. Additionally, the numerical calculations for this research were conducted on the supercomputing system at the Supercomputing Center of Wuhan University.
Author information
Authors and Affiliations
Contributions
G.Y. designed the project and directed the work. W.K., H.W., D.W. and P.L. conducted the experiments and data analysis. B.G. and Yuqiang Li performed all density functional theory calculations. G.Y., W.K., D.W. and Yangyang Li wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Albert Poater and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–8, Figs. 1–162, reaction development, experimental procedures, characterization data and computational details.
Supplementary Data 1
Coordinate files from the computational study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kong, W., Wu, D., Wei, H. et al. Head–tail carboboration of multisubstituted alkenes enabled by chain recognition. Nat. Chem. (2025). https://doi.org/10.1038/s41557-025-01903-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-025-01903-y