Abstract
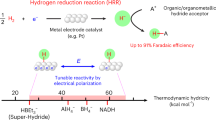
Heterolytic hydrogenations, which split H2 across a hydride acceptor and proton acceptor, comprise a key reaction class that spans the chemical value chain, including CO2 hydrogenation to formate and NADH regeneration from nicotinamide adenine dinucleotide (NAD+). The dominant mechanistic models for heterogeneous catalysis of these reactions invoke classical surface reaction steps, largely ignoring the role of interfacial charge separation. Here we quantify the electrochemical potential of the catalyst during turnover and uncover evidence supporting an interfacial electrochemical hydride transfer mechanism for this overall thermochemical reaction class. We find that the proton acceptor induces spontaneous electrochemical polarization of the metal catalyst surface, thereby controlling the thermodynamic hydricity of the surface M–H intermediates and driving rate-determining electrochemical hydride transfer to the hydride acceptor substrate. This mechanistic framework, which applies across diverse reaction media and for the hydrogenation of CO2 to formate and NAD+ to NADH, enables the determination of intrinsic reaction kinetics and exposes design principles for the future development of sustainable hydrogenation reactivity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are included in the published article (and its Supplementary Information) as .zip files or available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Becker, C. in Encyclopedia of Interfacial Chemistry (ed. Klaus Wandelt) 99–106 (Elsevier, 2018).
Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis (Wiley, 2001).
Álvarez, A. et al. Challenges in the greener production of formates/formic acid, methanol, and DME by heterogeneously catalyzed CO2 hydrogenation processes. Chem. Rev. 117, 9804–9838 (2017).
Qu, R., Junge, K. & Beller, M. Hydrogenation of carboxylic acids, esters, and related compounds over heterogeneous catalysts: a step toward sustainable and carbon-neutral processes. Chem. Rev. 123, 1103–1165 (2023).
Mattson, B. et al. Heterogeneous catalysis: the Horiuti–Polanyi mechanism and alkene hydrogenation. J. Chem. Edu. 90, 613–619 (2013).
Aireddy, D. R. & Ding, K. Heterolytic dissociation of H2 in heterogeneous catalysis. ACS Catal. 12, 4707–4723 (2022).
Wang, X. et al. Cofactor NAD(P)H regeneration inspired by heterogeneous pathways. Chem 2, 621–654 (2017).
Sun, R. et al. Heterogeneous catalysts for CO2 hydrogenation to formic acid/formate: from nanoscale to single atom. Energy Environ. Sci. 14, 1247–1285 (2021).
Ryu, J. & Surendranath, Y. Polarization-induced local pH swing promotes Pd-catalyzed CO2 hydrogenation. J. Am. Chem. Soc. 142, 13384–13390 (2020).
Wang, H.-X., Toh, W. L., Tang, B. Y. & Surendranath, Y. Metal surfaces catalyse polarization-dependent hydride transfer from H2. Nat. Catal. 6, 351–362 (2023).
Wesley, T. S., Román-Leshkov, Y. & Surendranath, Y. Spontaneous electric fields play a key role in thermochemical catalysis at metal-liquid interfaces. ACS Cent. Sci. 7, 1045–1055 (2021).
Wesley, T. S. et al. Metal nanoparticles supported on a nonconductive oxide undergo pH-dependent spontaneous polarization. Chem. Sci. 14, 7154–7160 (2023).
Popov, B. N. Corrosion Engineering: Principles and Solved Problems (Elsevier, 2015).
Fortunato, G. V. et al. Analysing the relationship between the fields of thermo- and electrocatalysis taking hydrogen peroxide as a case study. Nat. Commun. 13, 1973 (2022).
Adams, J. S., Kromer, M. L., Rodríguez-López, J. & Flaherty, D. W. Unifying concepts in electro- and thermocatalysis toward hydrogen peroxide production. J. Am. Chem. Soc. 143, 7940–7957 (2021).
Ryu, J. et al. Thermochemical aerobic oxidation catalysis in water can be analysed as two coupled electrochemical half-reactions. Nat. Catal. 4, 742–752 (2021).
An, H., Sun, G., Hülsey, M. J., Sautet, P. & Yan, N. Demonstrating the electron–proton-transfer mechanism of aqueous phase 4-nitrophenol hydrogenation using unbiased electrochemical Cells. ACS Catal. 12, 15021–15027 (2022).
Howland, W. C., Gerken, J. B., Stahl, S. S. & Surendranath, Y. Thermal hydroquinone oxidation on Co/N-doped carbon proceeds by a band-mediated electrochemical mechanism. J. Am. Chem. Soc. 144, 11253–11262 (2022).
Huang, X. et al. Au–Pd separation enhances bimetallic catalysis of alcohol oxidation. Nature 603, 271–275 (2022).
Lodaya, K. M. et al. An electrochemical approach for designing thermochemical bimetallic nitrate hydrogenation catalysts. Nat. Catal. 7, 262–272 (2024).
Kunnen, K., Nikonov, G. & Yunnikova, L. in Encyclopedia of Reagents in Organic Synthesis (eds Charette, A. et al.) 1–3 (Wiley, 2014).
Ilic, S., Gesiorski, J. L., Weerasooriya, R. B. & Glusac, K. D. Biomimetic metal-free hydride donor catalysts for CO2 reduction. Acc. Chem. Res. 55, 844–856 (2022).
Internet Bond-Energy Databank (Tsinghua Univ., 6 September 2025); https://ibond.las.ac.cn/
van der Plas, J. F., Barendrecht, E. & Zeilmaker, H. Electrocatalytic hydrogenation processes at controlled potential—2. Charge transfer at a slurry electrode. Electrochim. Acta 25, 1471–1475 (1980).
Ilic, S., Alherz, A., Musgrave, C. B. & Glusac, K. D. Thermodynamic and kinetic hydricities of metal-free hydrides. Chem. Soc. Rev. 47, 2809–2836 (2018).
Schmickler, W. & Santos, E. Interfacial Electrochemistry (Springer, 2010).
Sellés Vidal, L., Kelly, C. L., Mordaka, P. M. & Heap, J. T. Review of NAD(P)H-dependent oxidoreductases: properties, engineering and application. Biochim. Biophys. Acta Proteins Proteom. 1866, 327–347 (2018).
Wu, H. et al. Methods for the regeneration of nicotinamide coenzymes. Green Chem. 15, 1773–1789 (2013).
Wang, X. & Yiu, H. H. P. Heterogeneous catalysis mediated cofactor NADH regeneration for enzymatic reduction. ACS Catal. 6, 1880–1886 (2016).
Saba, T. et al. NADH regeneration: a case study of Pt-catalyzed NAD+ reduction with H2. ACS Catal. 11, 283–289 (2021).
Wang, M. et al. Chemoselective NADH regeneration: the synergy effect of TiOx and Pt in NAD+ hydrogenation. ACS Sustain.Chem. Eng 9, 6499–6506 (2021).
Burnett, J. W. H. et al. Directing the H2-driven selective regeneration of NADH via Sn-doped Pt/SiO2. Green Chem. 24, 1451–1455 (2022).
Kurimoto, A. et al. Bioelectrocatalysis with a palladium membrane reactor. Nat. Commun. 14, 1814 (2023).
Wiedner, E. S. et al. Thermodynamic hydricity of transition metal hydrides. Chem. Rev. 116, 8655–8692 (2016).
Rodkey, F. L. Oxidation-reduction potentials of the diphosphopyridine nucleotide system. J. Biol. Chem. 213, 777–786 (1955).
Brereton, K. R., Smith, N. E., Hazari, N. & Miller, A. J. M. Thermodynamic and kinetic hydricity of transition metal hydrides. Chem. Soc. Rev. 49, 7929–7948 (2020).
Smith, A. M. & Miller, A. J. M. Open circuit potential method for thermodynamic hydricity measurements of metal hydrides. Organometallics 43, 3163–3170 (2024).
Mikolajczyk, T., Luba, M., Pierozynski, B. & Smoczynski, L. A detrimental effect of acetonitrile on the kinetics of underpotentially deposited hydrogen and hydrogen evolution reaction, examined on Pt electrode in H2SO4 and NaOH solutions. Catalysts 10, 625 (2020).
Lowry, O. H., Passonneau, J. V. & Rock, M. K. The stability of pyridine nucleotides. J. Biol. Chem. 236, 2756–2759 (1961).
Dey, S., Masero, F., Brack, E., Fontecave, M. & Mougel, V. Electrocatalytic metal hydride generation using CPET mediators. Nature 607, 499–506 (2022).
Preiner, M. et al. A hydrogen-dependent geochemical analogue of primordial carbon and energy metabolism. Nat. Ecol. Evol. 4, 534–542 (2020).
Hudson, R. et al. CO2 reduction driven by a pH gradient. Proc. Natl Acad. Sci. USA 117, 22873–22879 (2020).
Bordet, A. et al. Selectivity control in hydrogenation through adaptive catalysis using ruthenium nanoparticles on a CO2-responsive support. Nat. Chem. 13, 916–922 (2021).
Chu, A. T., Jung, O., Toh, W. L. & Surendranath, Y. Organic non-nucleophilic electrolyte resists carbonation during selective CO2 electroreduction. J. Am. Chem. Soc. 145, 9617–9623 (2023).
DuBois, D. L. & Berning, D. E. Hydricity of transition-metal hydrides and its role in CO2 reduction. Appl. Organomet. Chem. 14, 860–862 (2000).
Waldie, K. M., Ostericher, A. L., Reineke, M. H., Sasayama, A. F. & Kubiak, C. P. Hydricity of transition-metal hydrides: thermodynamic considerations for CO2 reduction. ACS Catal. 8, 1313–1324 (2018).
Jin, W. et al. Catalytic upgrading of biomass model compounds: novel approaches and lessons learnt from traditional hydrodeoxygenation—a review. Chem. Cat. Chem. 11, 924–960 (2019).
Nelson, R. C. et al. Experimental and theoretical insights into the hydrogen-efficient direct hydrodeoxygenation mechanism of phenol over Ru/TiO2. ACS Catal. 5, 6509–6523 (2015).
Liu, P. et al. Photochemical route for synthesizing atomically dispersed palladium catalysts. Science 352, 797–800 (2016).
Cai, H., Schimmenti, R., Nie, H., Mavrikakis, M. & Chin, Y.-H. C. Mechanistic role of the proton–hydride pair in heteroarene catalytic hydrogenation. ACS Catal. 9, 9418–9437 (2019).
Bender, M. T., Lam, Y. C., Hammes-Schiffer, S. & Choi, K.-S. Unraveling two pathways for electrochemical alcohol and aldehyde oxidation on NiOOH. J. Am. Chem. Soc. 142, 21538–21547 (2020).
Ning, H. et al. Selective upgrading of biomass-derived benzylic ketones by (formic acid)–Pd/HPC–NH2 system with high efficiency under ambient conditions. Chem 7, 3069–3084 (2021).
Mahnaz, F. et al. Intermediate transfer rates and solid-state ion exchange are key factors determining the bifunctionality of In2O3/HZSM-5 tandem CO2 hydrogenation catalyst. ACS Sustain. Chem. Eng 12, 5197–5210 (2024).
Liu, F. et al. Electrocatalytic NAD+ reduction via hydrogen atom-coupled electron transfer. Chem. Sci. 13, 13361–13367 (2022).
Acknowledgements
We thank the entire Surendranath Laboratory for their support, with particular acknowledgement to T. Wesley, W. L. Toh and B. Y. Tang for fruitful discussion and H. W. Chung, D. Harraz and K. Westendorff for examining the paper. This research was supported by the National Science Foundation, under grant award number CHE-2400167 (Y.S.). H.-X.W. gratefully acknowledges support from the Croucher Fellowship.
Author information
Authors and Affiliations
Contributions
H.-X.W. and Y.S. conceived the research and developed experiments. H.-X.W. conducted the experiments. H.-X.W. and Y.S. analysed the data and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Ning Yan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–25, Discussion and Tables 1–4.
Supplementary Data 1
NMR data for BIM+ hydrogenation.
Supplementary Data 2
Open-circuit potential data for potential sensing experiments.
Supplementary Data 3
Open-circuit potential data and calculated hydricity values.
Supplementary Data 4
Comparison of open-circuit potential data for potential sensing experiments.
Supplementary Data 5
NMR data for BIM+ hydrogenation catalyzed by Pt/SiO2 using D2.
Supplementary Data 6
NMR data for BIM+ hydrogenation catalyzed by Pt/C using D2.
Supplementary Data 7
NMR data for BIM+ hydrogenation catalyzed by Pt/SiO2 using D2 in the absence of HOAc.
Supplementary Data 8
NMR spectral changes for BIM+ hydrogenation using MTBD.
Supplementary Data 9
Time course data and linear fits for BIM+ hydrogenation using various buffers.
Supplementary Data 10
Time course data and linear fits for BIM+ hydrogenation using various acid/base ratios.
Supplementary Data 12
Crude UV–Vis data for NAD+ hydrogenation at various pH values.
Supplementary Data 13
Crude UV–Vis data for NAD+ hydrogenation after treated with lipoamide dehydrogenase.
Supplementary Data 14
NMR data for H/D tracer experiment of NAD+.
Supplementary Data 15
UV–Vis spectral changes for NAD+ hydrogenation.
Supplementary Data 16
Time course data and linear fits for NAD+ hydrogenation.
Supplementary Data 17
Time course data, Tafel data and their linear fits for NAD+ hydrogenation.
Supplementary Data 18
NMR data for CO2 hydrogenation using various bases.
Supplementary Data 19
NMR data for H/D tracer reaction of CO2.
Supplementary Data 20
Time course data and linear fits for CO2 hydrogenation using various bases.
Supplementary Data 21
Time course data and linear fits for CO2 hydrogenation using various acid/base ratios.
Supplementary Data 22
Time course data, Tafel data and their linear fits for CO2 hydrogenation using Rh/C as catalyst.
Supplementary Data 23
Open-circuit potential data and calculated hydricity values for CO2 hydrogenation.
Supplementary Data 24
Open-circuit potential data for CO2 hydrogenation.
Source data
Source Data Fig. 2
Experimental data and linear fits used to plot Fig. 2b–e,g,h.
Source Data Fig. 4
Experimental data and linear fits used to plot Fig. 4c,d.
Source Data Fig. 5
Experimental data and linear fits used to plot Fig. 5c,d.
Source Data Fig. 6
Experimental data, linear fits and extrapolated data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, HX., Surendranath, Y. Thermochemical heterolytic hydrogenation catalysis proceeds through polarization-driven hydride transfer. Nat. Chem. 18, 43–50 (2026). https://doi.org/10.1038/s41557-025-01939-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41557-025-01939-0
This article is cited by
-
Electrochemistry lurks beneath the surface of thermocatalytic hydrogenations
Nature Chemistry (2026)