Abstract
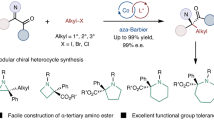
The development of synthetic methods that convert readily accessible chemicals into highly valuable small organic molecules, especially those with enantioenriched three-dimensional structures, holds substantial importance in organic synthesis. Alkyl halides are pivotal substrates for generating chiral C(sp3)–C(sp3) bonds. However, constructing modular vicinal stereogenic carbon centres from racemic alkyl halides, especially those bearing the sterically bulky quaternary carbon stereocentres frequently found in natural products, remains a daunting challenge. Here we report cobalt-catalysed enantioconvergent reductive addition of racemic alkyl halides with imines, allowing for efficient construction of various contiguous stereogenic centres, including tertiary–tertiary, tertiary–quaternary and quaternary–quaternary stereocentres with high diastereo- and enantioselectivities. The mild reaction conditions circumvent the use of organometallic reagents, ensuring broad functional group compatibility and enabling the formation of valuable chiral organic motifs such as amino acids, organophosphorus compounds, amino alcohols and γ-lactams. This reductive radical addition protocol also enables the stereoselective construction of C-glycosyl amino acids.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Crystallographic data for compounds 5, 33, 42, 78, 89, 98′, 102 and 124 are available from the Cambridge Crystallographic Data Center under reference numbers CCDC 2267486, 2339703, 2281055, 2347928, 2345237, 2348348, 2348347 and 2339794, respectively. All other data to support the conclusions are available in the main text or the supplementary materials.
References
Corey, E. J. & Kürti, L. Enantioselective Chemical Synthesis: Methods, Logic and Practice (Direct Book, 2010).
Lovering, F., Bikker, J. & Humblet, C. Escape from flatland: increasing saturation as an approach to improving clinical success. J. Med. Chem. 52, 6752–6756 (2009).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Enantioselective and enantiospecific transition-metal-catalyzed cross-coupling reactions of organometallic reagents to construct C–C bonds. Chem. Rev. 115, 9587–9652 (2015).
Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl–alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Yus, M., Nájera, C., Foubelo, F. & Sansano, J. M. Metal-catalyzed enantioconvergent transformations. Chem. Rev. 123, 11817–11893 (2023).
Fischer, C. & Fu, G. C. Asymmetric nickel-catalyzed Negishi cross-couplings of secondary α-bromo amides with organozinc reagents. J. Am. Chem. Soc. 127, 4594–4595 (2005).
Mao, J. et al. Cobalt-bisoxazoline-catalyzed asymmetric Kumada cross-coupling of racemic α-bromo esters with aryl Grignard reagents. J. Am. Chem. Soc. 136, 17662–17668 (2014).
Jiang, S.-P. et al. Copper-catalyzed enantioconvergent radical Suzuki–Miyaura C(sp3)–C(sp2) cross-coupling. J. Am. Chem. Soc. 142, 19652–19659 (2020).
Wang, F.-L. et al. Mechanism-based ligand design for copper-catalysed enantioconvergent C(sp3)–C(sp) cross-coupling of tertiary electrophiles with alkynes. Nat. Chem. 14, 949–957 (2022).
Cherney, A. H., Kadunce, N. T. & Reisman, S. E. Catalytic asymmetric reductive acyl cross-coupling: synthesis of enantioenriched acyclic α,α-disubstituted ketones. J. Am. Chem. Soc. 135, 7442–7445 (2013).
Wang, Z., Yin, H. & Fu, G. C. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature 563, 379–383 (2018).
Zhao, W.-T. & Shu, W. Enantioselective Csp3–Csp3 formation by nickel-catalyzed enantioconvergent cross-electrophile alkyl–alkyl coupling of unactivated alkyl halides. Sci. Adv. 9, eadg9898 (2023).
Huo, H., Gorsline, B. J. & Fu, G. C. Catalyst-controlled doubly enantioconvergent coupling of racemic alkyl nucleophiles and electrophiles. Science 367, 559–564 (2020).
An, L., Tong, F.-F., Zhang, S. & Zhang, X. Stereoselective functionalization of racemic cyclopropylzinc reagents via enantiodivergent relay coupling. J. Am. Chem. Soc. 142, 11884–11892 (2020).
Feng, J., Holmes, M. & Krische, M. J. Acyclic quaternary carbon stereocenters via enantioselective transition metal catalysis. Chem. Rev. 117, 12564–12580 (2017).
Pierrot, D. & Marek, I. Synthesis of enantioenriched vicinal tertiary and quaternary carbon stereogenic centers within an acyclic chain. Angew. Chem. Int. Ed. 59, 36–49 (2020).
Tian, Q. & Zhang, G. Recent advances in the asymmetric Nozaki–Hiyama–Kishi reaction. Synthesis 48, 4038–4049 (2016).
Gil, A., Albericio, F. & Álvarez, M. Role of the Nozaki–Hiyama–Takai–Kishi reaction in the synthesis of natural products. Chem. Rev. 117, 8420–8446 (2017).
Bandini, M., Cozzi, P. G. & Umani-Ronchi, A. Salen as a chiral activator: anti versus syn switchable diastereoselection in the enantioselective addition of crotyl bromide to aromatic aldehydes. Angew. Chem. Int. Ed. 39, 2327–2330 (2000).
Kurosu, M., Lin, M.-H. & Kishi, Y. Fe/Cr- and Co/Cr-mediated catalytic asymmetric 2-haloallylations of aldehydes. J. Am. Chem. Soc. 126, 12248–12249 (2004).
Zhang, S. et al. Design and synthesis of tunable chiral 2,2′-bipyridine ligands: application to the enantioselective nickel-catalyzed reductive arylation of aldehydes. Angew. Chem. Int. Ed. 61, e202117843 (2022).
Zhu, Z., Xiao, J., Li, M. & Shi, Z. Nickel-catalyzed intermolecular asymmetric addition of aryl iodides across aldehydes. Angew. Chem. Int. Ed. 61, e202201370 (2022).
Jiang, X. et al. Photoassisted cobalt-catalyzed asymmetric reductive Grignard-type addition of aryl iodides. J. Am. Chem. Soc. 144, 8347–8354 (2022).
Zhang, L. et al. Nickel-catalyzed enantioselective reductive arylation and heteroarylation of aldimines via an elementary 1,4-addition. J. Am. Chem. Soc. 145, 8498–8509 (2023).
Liang, T. et al. Visible light-mediated cobalt and photoredox dual-catalyzed asymmetric reductive coupling for axially chiral secondary alcohols. Chin. J. Chem. 41, 3253–3260 (2023).
Kumar, R., Flodén, N. J., Whitehurst, W. G. & Gaunt, M. J. A general carbonyl alkylative amination for tertiary amine synthesis. Nature 581, 415–420 (2020).
Turro, R. F., Brandstätter, M. & Reisman, S. E. Nickel-catalyzed reductive alkylation of heteroaryl imines. Angew. Chem. Int. Ed. 61, e202207597 (2022).
Wu, X. et al. Modular α-tertiary amino ester synthesis through cobalt-catalysed asymmetric aza-Barbier reaction. Nat. Chem. 16, 398–407 (2024).
Gao, Y. et al. Electrocatalytic asymmetric Nozaki–Hiyama–Kishi decarboxylative coupling: scope, applications, and mechanism. J. Am. Chem. Soc. 146, 4872–4882 (2024).
Shibasaki, M. & Kanai, M. Asymmetric synthesis of tertiary alcohols and α-tertiary amines via Cu-catalyzed C–C bond formation to ketones and ketimines. Chem. Rev. 108, 2853–2873 (2008).
Ruan, L.-X., Sun, B., Liu, J.-M. & Shi, S.-L. Dynamic kinetic asymmetric arylation and alkenylation of ketones. Science 379, 662–670 (2023).
Subramanian, H., Landais, Y. & Sibi, M. P. in Comprehensive Organic Synthesis Vol. II (eds Knochel, P. & Molander, G. A.) 699–741 (Elsevier, 2014).
Mondal, S. et al. Enantioselective radical reactions using chiral catalysts. Chem. Rev. 122, 5842–5976 (2022).
Pitzer, L., Schwarz, J. L. & Glorius, F. Reductive radical-polar crossover: traditional electrophiles in modern radical reactions. Chem. Sci. 10, 8285–8291 (2019).
He, X.-K. et al. Desymmetrization–addition reaction of cyclopropenes to imines via synergistic photoredox and cobalt catalysis. J. Am. Chem. Soc. 146, 18892–18898 (2024).
Li, Y., Lei, M. & Gong, L. Photocatalytic regio- and stereoselective C(sp3)–H functionalization of benzylic and allylic hydrocarbons as well as unactivated alkanes. Nat. Catal. 2, 1016–1026 (2019).
Biegasiewicz, K. F. et al. Photoexcitation of flavoenzymes enables a stereoselective radical cyclization. Science 364, 1166–1169 (2019).
Cheng, L. et al. Stereoselective amino acid synthesis by synergistic photoredox-pyridoxal radical biocatalysis. Science 381, 444–451 (2023).
Hou, L. et al. Enantioselective radical addition to ketones through Lewis acid-enabled photoredox catalysis. J. Am. Chem. Soc. 144, 22140–22149 (2022).
Song, X. et al. Catalytic asymmetric synthesis of azaarene-functionalized tertiary amines and α-amino acid derivatives from E/Z-ketimine mixtures via enantioselective radical coupling. ACS Catal. 13, 6396–6402 (2023).
Gosmini, C. & Auffrant, A. in Patai’s Chemistry of Functional Groups (Wiley, 2022).
Presset, M. et al. CoI-catalyzed Barbier reactions of aromatic halides with aromatic aldehydes and imines. Chem. Eur. J. 49, 4491–4495 (2019).
Li, Y. et al. Cobalt-catalysed enantioselective C(sp3)–C(sp3) coupling. Nat. Catal. 4, 901–911 (2021).
Zhou, F. et al. Catalytic enantioselective construction of vicinal quaternary carbon stereocenters. Chem. Sci. 11, 9341–9365 (2020).
Nugent, T. C. (ed.) Chiral Amine Synthesis: Methods, Developments and Applications (Wiley-VCH, 2010).
Blaskovich, M. A. T. Unusual amino acids in medicinal chemistry. J. Med. Chem. 59, 10807–10836 (2016).
Wu, L. et al. Anionic bisoxazoline ligands enable copper-catalyzed asymmetric radical azidation of acrylamides. Angew. Chem. Int. Ed. 60, 6997–7001 (2021).
Chen, J.-J. et al. Enantioconvergent Cu-catalysed N-alkylation of aliphatic amines. Nature 618, 294–300 (2023).
Li, P. et al. Stereodivergent access to non-natural α-amino acids via enantio- and Z/E-selective catalysis. Science 385, 972–979 (2024).
Kolodiazhnyi, O. I., Kukhar, V. P. & Kolodiazhna, A. O. Asymmetric catalysis as a method for the synthesis of chiral organophosphorus compounds. Tetrahedron Asymmetry 25, 865–922 (2014).
Ye, L.-W., Shu, C. & Gagosz, F. Recent progress towards transition metal-catalyzed synthesis of γ-lactams. Org. Biomol. Chem. 12, 1833–1845 (2014).
Dondoni, A. & Marra, A. Methods for anomeric carbon-linked and fused sugar amino acid synthesis: the gateway to artificial glycopeptides. Chem. Rev. 100, 4395–4422 (2000).
Ghorai, S., Chirke, S. S., Xu, W.-B., Chen, J.-F. & Li, C. Cobalt-catalyzed regio- and enantioselective allylic amination. J. Am. Chem. Soc. 141, 11430–11434 (2019).
Chen, A., Yang, B., Zhou, Z. & Zhu, F. Recent advances in transition-metal-catalyzed glycosyl cross-coupling reactions. Chem. Catal. 2, 3430–3470 (2022).
Shang, W., Shi, R. & Niu, D. C-glycoside synthesis enabled by nickel catalysis. Chin. J. Chem. 41, 2217–2236 (2023).
Wu, K. J. Y. et al. An antibiotic preorganized for ribosomal binding overcomes antimicrobial resistance. Science 383, 721–726 (2024).
Shang, M. et al. Modular, stereocontrolled Cβ–H/Cα–C activation of alkyl carboxylic acids. Proc. Natl Acad. Sci. USA 116, 8721–8727 (2019).
Acknowledgements
We thank the Analysis and Testing Center of East China University of Science and Technology for help with NMR and high-resolution mass spectrometry analysis. This work was supported by the National Natural Science Foundation of China (22171079, 22371071, 92356301, T2488302, 22471191, 2240011806), the Science and Technology Commission of Shanghai Municipality (grant number 24DX1400200), the Program of Introducing Talents of Discipline to Universities (B16017), the Scientific Committee of Shanghai (21JC1401700), the Shanghai Sailing Program (23YF1408800) and the Fundamental Research Funds for the Central Universities.
Author information
Authors and Affiliations
Contributions
Y.C. conceived of the project. X.W., T.X., J.B., C.F., J.H., W.W., C.Z. and Q.W. performed the experiments under the supervision of J.Q. and Y.C. Y.S. performed DFT calculations under the supervision of G.H. X.W., G.H. and Y.C. wrote the paper with feedback from all the other authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Experimental mechanistic studies.
a, Radical clock experiment. b, Tracking the enantiopurity of the secondary α-chloroamides. c, Stereodivergent synthesis of 3. Standard condition: 1a (1.0 equiv.), 2 (1.5 equiv.), CoI2 (5 mol%), L4 (6 mol%), In (2.0 equiv.), EtOH (1.0 equiv.) in MeCN/THF (v/v = 1/1, 0.2 M) under 35 °C for 48 h. Isolated yields were reported, the e.e. was determined by chiral HPLC analysis and d.r. was determined by crude NMR.
Extended Data Fig. 2 DFT Calculations and proposed catalytic cycle.
a, C-Cl bond cleavage using IM1triplet as the active catalyst species. b, C-Cl bond cleavage and C-C bond formation using IM3d°ublet as the active catalyst species. c, Proposed catalytic cycle. The Gibbs free energies were computed at the level of (u)ωB97X-D4(SMD)/ZORA-def2-TZVPP//(u)BP86(SMD)/6-31 G(d)&SDD. R and S denote the stereochemical configuration of the product.
Extended Data Fig. 3 Origins of Stereoselectivity.
a, Optimized geometries and non-covalent interaction analysis of radical addition transition states. b, Structural analysis of IM1triplet. c, Distortion/interaction analysis of radical addition transition states. ΔE‡int: interaction energy, ΔE‡dist[2a’]: distortion energy of 2a’, ΔE‡dist[IM1triplet]: distortion energy of IM1triplet.
Supplementary information
Supplementary Information
Supplementary Figs. 1–24 and Tables 1–8.
Supplementary Data 1
Cartesian coordinates.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, X., Xia, T., Bai, J. et al. Enantioconvergent radical addition of racemic alkyl halides to access vicinal stereocentres. Nat. Chem. (2025). https://doi.org/10.1038/s41557-025-01967-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-025-01967-w