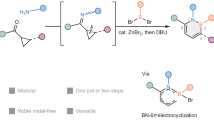
Abstract
In drug discovery processes, changing the core structures of lead compounds to a variety of other ring systems is often needed, which typically requires laborious de novo syntheses of individual analogues. Here we report a conceptually different approach that allows rapid access to diverse core structures from a common intermediate using 1,2-oxaborines as a versatile molecular platform. A soft enolization/6π-electrocyclization strategy has been developed to efficiently synthesize 1,2-oxaborines from readily available enones or enals. Taking advantage of their multifaceted reactivities, 1,2-oxaborines can undergo further C−H functionalization and be transformed into a diverse range of arenes, heteroarenes and non-aromatic heterocycles. Finally, late-stage preparations of a suite of analogues that contain Lipitor substituents but with different aromatic cores are demonstrated using the 1,2-oxaborine-based core diversification strategy.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All the data generated or analysed during this study are included in this Article and its Supplementary Information. Crystallographic data for the structure of 3ja reported in this study has been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition number 2409545. Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures.
References
Hu, Y. & Bajorath, J. Many drugs contain unique scaffolds with varying structural relationships to scaffolds of currently available bioactive compounds. Eur. J. Med. Chem. 76, 427–434 (2014).
Kombarov, R. et al. BioCores: identification of a drug/natural product-based privileged structural motif for small-molecule lead discovery. Mol. Divers. 14, 193–200 (2010).
Catapano, A. L. Pitavastatin: a different pharmacological profile. Clin. Lipidol. 7, 3–9 (2012).
Turner, N. A., Midgley, L., O’Regan, D. J. & Porter, K. E. Comparison of the efficacies of five different statins on inhibition of human saphenous vein smooth muscle cell proliferation and invasion. J. Cardiovasc. Pharmacol. 50, 458–461 (2007).
Yamada, K., Matsuki, K., Omori, K., & Kikkawa, K. Cyclic compounds. WO Patent 2001083460A1 (2001).
Yamada, K., Sakamoto, T., Omori, K. & Kikkawa, K. Successful Drug Discovery (eds Fischer, J. & Rotella, D.) 71–76 (Wiley, 2015).
Li, E.-Q., Lindsley, C. W., Chang, J. B. & Yu, B. Molecular skeleton editing for new drug discovery. J. Med. Chem. 67, 13509–13511 (2024).
Joynson, B. W. & Ball, L. T. Skeletal editing: interconversion of arenes and heteroarenes. Helv. Chim. Acta 106, e202200182 (2023).
Jurczyk, J. et al. Single-atom logic for heterocycle editing. Nat. Synth. 1, 352–364 (2022).
Liu, Z., Sivaguru, P., Ning, Y., Wu, Y. & Bi, X. Skeletal editing of (hetero)arenes using carbenes. Chem. Eur. J. 29, e202301227 (2023).
Cheng, Q. et al. Skeletal editing of pyridines through atom-pair swap from CN to CC. Nat. Chem. 16, 741–748 (2024).
Wu, F.-P. et al. Ring expansion of indene by photoredox-enabled functionalized carbon-atom insertion. Nat. Catal. 7, 242–251 (2024).
Finkelstein, P. et al. Nitrogen atom insertion into indenes to access isoquinolines. Chem. Sci. 14, 2954–2959 (2023).
Li, C. et al. C-F bond insertion into indoles with CHBr2F: an efficient method to synthesize fluorinated quinolines and quinolones. Chin. J. Chem. 42, 1128–1132 (2024).
Roure, B. et al. Photochemical permutation of thiazoles, isothiazoles and other azoles. Nature 637, 860–867 (2025).
McConnell, C. R. & Liu, S.-Y. Late-stage functionalization of BN-heterocycles. Chem. Soc. Rev. 48, 3436–3453 (2019).
Giustra, Z. X. & Liu, S.-Y. The state of the art in azaborine chemistry: new synthetic methods and applications. J. Am. Chem. Soc. 140, 1184–1194 (2018).
Campbell, P. G., Marwitz, A. J. V. & Liu, S.-Y. Recent advances in azaborine chemistry. Angew. Chem. Int. Ed. 51, 6074–6092 (2012).
Su, W. L. et al. Copper-catalysed asymmetric hydroboration of alkenes with 1,2-benzazaborines to access chiral naphthalene isosteres. Nat. Chem. 16, 1312–1319 (2024).
Zhao, P., Nettleton, D. O., Karki, R. G., Zécri, F. J. & Liu, S.-Y. Medicinal chemistry profiling of monocyclic 1,2-azaborines. ChemMedChem 12, 358–361 (2017).
Chen, M. et al. A BN-doped cycloparaphenylene debuts. Angew. Chem. Int. Ed. 60, 1556–1560 (2021).
Chen, J. H., Bajko, Z., Kampf, J. W. & Ashe, A. J. III Organometallics 26, 1563–1564 (2007).
Yruegas, S., Pattersona, D. C. & Martin, C. D. Oxygen insertion into boroles as a route to 1,2-oxaborines. Chem. Commun. 52, 6658–6661 (2016).
Nava, N. A. 1,2-Oxaborines: Synthesis, Properties, and Reactivity. Master’s Thesis, Boston College (2017).
Köster, R. & Pourzal, A.-A. Kondensationsprodukte von alkyl-phenylketonen. Synthesis 11, 674–676 (1973).
Lyu, H. et al. Modular synthesis of 1,2-azaborines via ring-opening BN-isostere benzannulation. Nat. Chem. 16, 269–276 (2024).
Choi, S. & Dong, G. Rapid and modular access to multifunctionalized 1,2-azaborines via palladium/norbornene cooperative catalysis. J. Am. Chem. Soc. 146, 9512–9518 (2024).
Casey, C. P., Jones, C. R. & Tukada, H. Interconversion of γ-siIyl α,β-unsaturated carbonyl compounds and siloxybutadienes by 1,5-shifts of silicon between carbon and oxygen. J. Org. Chem. 46, 2089–2092 (1981).
Braun, M. Modern Aldol Reactions (ed. Mahrwald, R.) 1–61 (Wiley, 2004).
Baggett, A. W., Vasiliu, M., Li, B., Dixon, D. A. & Liu, S.-Y. Late-stage functionalization of 1,2-dihydro-1,2-azaborines via regioselective iridium-catalyzed C–H borylation: the development of a new N,N-bidentate ligand scaffold. J. Am. Chem. Soc. 137, 5536–5541 (2015).
Pan, J., Kampf, J. W. & Ashe, A. J. Electrophilic aromatic substitution reactions of 1,2-dihydro-1,2-azaborines. Org. Lett. 9, 679–681 (2007).
Brown, A. N., Li, B. & Liu, S.-Y. Negishi cross-coupling is compatible with a reactive B–Cl bond: development of a versatile late stage functionalization of 1,2-azaborines and its application to the synthesis of new BN isosteres of naphthalene and indenyl. J. Am. Chem. Soc. 137, 8932–8935 (2015).
Brown, A. N., Li, B. & Liu, S.-Y. Expanding the functional group tolerance of cross-coupling in 1,2-dihydro-1,2-azaborines: installation of alkyl, alkenyl, aryl, and heteroaryl substituents while maintaining a B–H bond. Tetrahedron 75, 580–583 (2019).
Sasaki, M., Hamzik, P. J., Ikemoto, H., Bartko, S. G. & Danheiser, R. L. Formal bimolecular [2 + 2 + 2] cycloaddition strategy for the synthesis of pyridines: intramolecular propargylic Ene reaction/Aza Diels–Alder reaction cascades. Org. Lett. 20, 6244–6249 (2018).
Lee, J. J., Pollock, G. R., Mitchell, D., Kasuga, L. & Kraus, G. A. Upgrading malic acid to bio-based benzoates via a Diels–Alder-initiated sequence with the methyl coumalate platform. RSC Adv. 4, 45657–45664 (2014).
Liu, J. et al. Photoredox-enabled chromium-catalyzed alkene diacylations. ACS Catal. 12, 1879–1885 (2022).
Üsküp, H. C., Yıldız, T., Onar, H. Ç & Hasdemir, B. Synthesis of novel 1,4-diketone derivatives and their further cyclization. ACS Omega 8, 14047–14052 (2023).
Thilagavathi, R. et al. Three-dimensional quantitative structure (3-D QSAR) activity relationship studies on imidazolyl and N-pyrrolyl heptenoates as 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR) inhibitors by comparative molecular similarity indices analysis (CoMSIA). Bioorg. Med. Chem. Lett. 15, 1027–1032 (2005).
Sakaguchi, H. et al. Copper-catalyzed regioselective monodefluoroborylation of polyfluoroalkenes en route to diverse fluoroalkenes. J. Am. Chem. Soc. 139, 12855–12862 (2017).
Acknowledgements
The University of Chicago is acknowledged for research support. We thank A. S. Filatov (University of Chicago) and X. Liu (University of Chicago) for X-ray crystallography. K. Wen (University of Chicago) is thanked for checking the experimental procedure.
Author information
Authors and Affiliations
Contributions
G.D. and Y.G. conceived and designed the experiments. Y.G., Q.Z and Y.Z. performed the experiments and analysed the data. Y.G. and G.D. prepared the paper together. G.D. directed the research.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks Adam Noble, Qiuling Song and Tülay Yıldız for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–4, discussion and Tables 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ge, Y., Zhu, Q., Zhu, Y. et al. Core diversification using 1,2-oxaborines as a versatile molecular platform. Nat. Chem. (2025). https://doi.org/10.1038/s41557-025-01971-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41557-025-01971-0