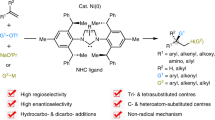
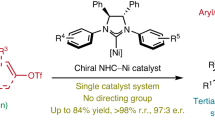
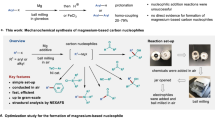
Abstract
Grignard reagents—cornerstones of synthetic chemistry—are hindered by enduring limitations in accessing complex architectures, which poses a persistent synthetic bottleneck. Meanwhile, quaternary carbon (stereo)centres, ubiquitous in bioactive molecules and natural products, remain formidable synthetic targets despite decades of research. Here we introduce a nickel-catalysed carbomagnesiation strategy that simultaneously overcomes these challenges through a rare contra-electronegativity transmetallation (Ni to Mg). This approach enables the efficient and modular synthesis of β-quaternary Grignard reagents via carbomagnesiation of 1,1-disubstituted alkenes and 1,3-dienes, employing aryl triflate and PhMgBr as carbon and magnesium sources, respectively. The resulting organomagnesium reagents undergo one-pot reactions with diverse electrophiles, delivering stereochemically complex quaternary centres with high precision. Mechanistically, bulky N-heterocyclic carbene (NHC)-based catalysts divert classical cross-coupling pathways, enforcing a counterintuitive Ni-to-Mg transmetallation that defies conventional electronegativity trends while achieving exceptional regio- and enantiocontrol. This contra-electronegativity transmetallation demonstrates substantial potential to advance carbometallation reactions and open new avenues for cross-coupling chemistry.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the Article and its Supplementary Information. Crystallographic data for the structures reported in this Article have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition no. 2417329 (12d). Copies of the data can be obtained free of charge via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this Paper.
References
Grignard, V. Some new organometallic combinations of magnesium and their application to the synthesis of alcohols and hydrocarbons. C. R. Acad. Sci. 130, 1322–1325 (1900).
Silverman, G. S. & Rakita, P. E. (eds) Handbook of Grignard Reagents (Marcel Dekker, 1996).
Knappke, C. E. I. & Wangelin, A. J. V. 35 years of palladium-catalyzed cross-coupling with Grignard reagents: how far have we come?. Chem. Soc. Rev. 40, 4948–4962 (2011).
Banno, T., Hayakawa, Y. & Umeno, M. Some applications of the Grignard cross-coupling reaction in the industrial field. J. Organomet. Chem. 653, 288–291 (2022).
Walborsky, H. M. Mechanism of Grignard reagent formation. The surface nature of the reaction. Acc. Chem. Res. 23, 286–293 (1990).
Knochel, P. et al. Highly functionalized organomagnesium reagents prepared through halogen-metal exchange. Angew. Chem. Int. Ed. 42, 4302–4432 (2003).
Dzhemilev, U. M., Vostrikova, O. S. & Tolstikov, G. A. Homogeneous zirconium based catalysts in organic synthesis. J. Organomet. Chem. 304, 17–39 (1986).
Murakami, K. & Yorimitsu, H. Recent advances in transition-metal-catalyzed intermolecular carbomagnesiation and carbozincation. Beilstein J. Org. Chem. 9, 278–302 (2013).
Müllera, D. S. & Marek, I. Copper mediated carbometalation reactions. Chem. Soc. Rev. 45, 4552–4566 (2016).
Cohen, Y. & Marek, I. Regio- and diastereoselective carbometalation reaction of cyclopropenes. Acc. Chem. Res. 55, 2848–2868 (2022).
Hoveyda, A. H. & Xu, Z. Stereoselective formation of carbon-carbon bonds through metal catalysis. The zirconium-catalyzed ethylmagnesiation reaction. J. Am. Chem. Soc. 113, 5079–5080 (1991).
Knight, K. S. & Waymouth, R. M. The zirconium-catalyzed ethylmagnesiation reaction, Zirconium-catalyzed diene and alkyl-alkene coupling reactions with magnesium reagents. J. Am. Chem. Soc. 113, 6268–6270 (1991).
Takahashi, T. et al. Remarkably ‘Pair’ selective and regioselective carbon-carbon bond forming reaction of zirconacylclopentane derivatives with Grignard reagents. J. Am. Chem. Soc. 113, 6266–6268 (1991).
Nakamura, M., Hirai, A. & Nakamura, E. Iron-catalyzed olefin carbometalation. J. Am. Chem. Soc. 122, 978–979 (2000).
Liao, L. & Fox, J. M. A copper-catalyzed method for the facially selective addition of Grignard reagents to cyclopropenes. J. Am. Chem. Soc. 124, 14322–14323 (2002).
de Armas, J. & Hoveyda, A. H. Zr-catalyzed electrophilic carbomagnesation of aryl olefins. Mechanism-based control of Zr-Mg ligand exchange. Org. Lett. 3, 2097–2100 (2001).
Dian, L., Muller, D. S. & Marek, I. Asymmetric copper-catalyzed carbomagnesiation of cyclopropenes. Angew. Chem. Int. Ed. 56, 6783–6787 (2017).
Morken, J. P., Didiuk, M. T. & Hoveyda, A. H. Zirconium-catalyzed asymmetric carbomagnesation. J. Am. Chem. Soc. 115, 6997–6998 (1993).
Didiuk, M. T., Johannes, C. W., Morken, J. P. & Hoveyda, A. H. Enantio-, diastereo- and regioselective zirconium-catalyzed carbomagnesation of cyclic ethers with higher alkyls of magnesium. Utility in synthesis and mechanistic implications. J. Am. Chem. Soc. 117, 7097–7104 (1995).
Christoffers, J. & Baro, A. (eds) Quaternary Stereocenters: Challenges and Solutions for Organic Synthesis (Wiley, 2005).
Feng, J.-J., Holmes, M. & Krische, M. J. Acyclic quaternary carbon stereocenters via enantioselective transition metal catalysis. Chem. Rev. 117, 12564–12580 (2017).
Wang, Z.-B., Yin, H.-L. & Fu, G. C. Catalytic enantioconvergent coupling of secondary and tertiary electrophiles with olefins. Nature 563, 379–383 (2018).
Mei, T.-S., Patel, H. H. & Sigman, M. S. Enantioselective construction of remote quaternary stereocentres. Nature 508, 340–344 (2014).
Huang, W. et al. Palladium-catalyzed enantioselective multicomponent cross-coupling of trisubstituted olefins. J. Am. Chem. Soc. 146, 16892–16901 (2024).
Liu, C.-F. et al. Synthesis of tri- and tetrasubstituted stereocentres by nickel-catalysed enantioselective olefin cross-couplings. Nat. Catal. 5, 934–942 (2022).
Luo, X. et al. Enantioselective synthesis of multifunctional alkylboronates via N-heterocyclic carbene–nickel-catalysed carboboration of alkenes. Nat. Synth. 3, 633–642 (2024).
Zhang, W.-B., Chen, G. & Shi, S.-L. Enantioselective Ni/N-heterocyclic carbene-catalyzed redox-economical coupling of aldehydes, alkynes, and enones for rapid construction of acyclic all-carbon quaternary stereocenters. J. Am. Chem. Soc. 144, 130–136 (2022).
Wang, Z.-C., Gao, L., Liu, S.-Y., Wang, P. & Shi, S.-L. Facile access to quaternary carbon centers via Ni-catalyzed arylation of alkenes with organoborons. J. Am. Chem. Soc. 147, 3023–3031 (2025).
de Meijere, A. & Diederich, F. (eds) Metal-Catalyzed Cross-Coupling Reactions 2nd edn (Wiley, 2004).
Yu, D.-G. et al. Direct arylation/alkylation/magnesiation of benzyl alcohols in the presence of Grignard reagents via Ni-, Fe- or Co-catalyzed sp3 C-O bond activation. J. Am. Chem. Soc. 134, 14638–14641 (2012).
Sun, T. et al. Nickel-catalyzed chemoselective carbomagnesiation for atroposelective ring-opening difunctionalization. Angew. Chem. Int. Ed. 63, e202401756 (2024).
Cai, Y. et al. Copper-catalyzed enantioselective Markovnikov protoboration of α-olefins enabled by a buttressed N-heterocyclic carbene ligand. Angew. Chem. Int. Ed. 57, 1376–1380 (2018).
Ruan, L.-X., Sun, B., Liu, J.-M. & Shi, S.-L. Dynamic kinetic asymmetric arylation and alkenylation of ketones. Science 379, 662–670 (2023).
Wang, Z.-C. et al. Enantioselective C–C cross-coupling of unactivated alkenes. Nat. Catal. 6, 1087–1097 (2023).
Wang, Z.-C. & Shi, S.-L. Induced-fit chiral N-heterocyclic carbene ligands for asymmetric catalysis. Acc. Chem. Res. 58, 2157–2177 (2025).
Qi, X.-X. & Diao, T.-N. Nickel-catalyzed dicarbofunctionalization of alkenes. ACS Catal. 10, 8542–8556 (2020).
Wickham, L. M. & Giri, R. Transition metal (Ni, Cu, Pd)-catalyzed alkene dicarbofunctionalization reactions. Acc. Chem. Res. 54, 3415–3437 (2021).
Peltzer, R. M., Eisenstein, O., Nova, A. & Cascella, M. How solvent dynamics controls the Schlenk equilibrium of Grignard reagents: a computational study of CH3MgCl in tetrahydrofuran. J. Phys. Chem. B 121, 4226–4237 (2017).
Wang, H. et al. Directing-group-free catalytic dicarbofunctionalization of unactivated alkenes. Nat. Chem. 14, 188–195 (2022).
Acknowledgements
This work is supported by the National Key R&D Program of China (2022YFA1503702), the National Natural Science Foundation of China (22325110, 92256303, 22171280), the Strategic Priority Research Program of the CAS (XDB0610000, XDA0540000), and the CAS Youth Interdisciplinary Team (JCTD-2021-11). We thank Y. Wang for helpful discussion of the manuscript.
Author information
Authors and Affiliations
Contributions
S.-L.S. conceived and directed the projects. X.Y. and B.S. performed the experiments. All authors analysed the data. S.-L.S. and B.S. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemistry thanks David Nelson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1–5 and Fig. 1
Source data
Source Data Fig. 3
Reaction profile—Fig. 3d
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, X., Sun, B. & Shi, SL. Contra-electronegativity transmetallation unlocks alkene carbomagnesiation to access quaternary stereocentres. Nat. Chem. (2026). https://doi.org/10.1038/s41557-026-02073-1
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41557-026-02073-1