Abstract
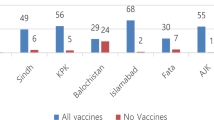
Vaccines that autonomously transfer among individuals have been proposed as a strategy to control infectious diseases within inaccessible wildlife populations. However, rates of vaccine spread and epidemiological efficacy in real-world systems remain elusive. Here, we investigate whether topical vaccines that transfer among individuals through social contacts can control vampire bat rabies—a medically and economically important zoonosis in Latin America. Field experiments in three Peruvian bat colonies, which used fluorescent biomarkers as a proxy for the bat-to-bat transfer and ingestion of an oral vaccine, revealed that vaccine transfer would increase population-level immunity up to 2.6 times beyond the same effort using conventional, non-spreadable vaccines. Mathematical models showed that observed levels of vaccine transfer would reduce the probability, size and duration of rabies outbreaks, even at low but realistically achievable levels of vaccine application. Models further predicted that existing vaccines provide substantial advantages over culling bats—the policy currently implemented in North, Central and South America. Linking field studies with biomarkers to mathematical models can inform how spreadable vaccines may combat pathogens of health and conservation concern before costly investments in vaccine design and testing.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data for Figs. 1 and 2 are provided with this Article at https://doi.org/10.1038/s41559-019-1032-x. The complete dataset describing ultraviolet and RB transfer is available from Dryad (https://doi.org/10.5061/dryad.64t161m).
Code availability
References
Daszak, P., Cunningham, A. A. & Hyatt, A. D. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287, 443–449 (2000).
Brochier, B. et al. Large-scale eradication of rabies using recombinant vaccinia-rabies vaccine. Nature 354, 520–522 (1991).
Slate, D. et al. Oral rabies vaccination in North America: opportunities, complexities, and challenges. PLoS Negl. Trop. Dis. 3, e549 (2009).
Hoffmann, A. et al. Successful establishment of Wolbachia in Aedes populations to suppress dengue transmission. Nature 476, 454–457 (2011).
Sinkins, S. P. & Gould, F. Gene drive systems for insect disease vectors. Nat. Rev. Genet. 7, 427–435 (2006).
Aliota, M. T., Peinado, S. A., Velez, I. D. & Osorio, J. E. The wMel strain of Wolbachia reduces transmission of Zika virus by Aedes aegypti. Sci. Rep. 6, 28792 (2016).
Basinski, A. J. et al. Evaluating the promise of recombinant transmissible vaccines. Vaccine 36, 675–682 (2018).
Bull, J. J., Smithson, M. W. & Nuismer, S. L. Transmissible viral vaccines. Trends Microbiol. 26, 6–15 (2018).
Minor, P. Vaccine-derived poliovirus (VDPV): impact on poliomyelitis eradication. Vaccine 27, 2649–2652 (2009).
Behdenna, A. et al. Transmission ecology of canine parvovirus in a multi-host, multi-pathogen system. Proc. R. Soc. B Biol. Sci. 286, 20182772 (2019).
Nuismer, S. L. et al. Eradicating infectious disease using weakly transmissible vaccines. Proc. R. Soc. Lond. B Biol. Sci. 283, 20161903 (2016).
Torres, J. M. et al. First field trial of a transmissible recombinant vaccine against myxomatosis and rabbit hemorrhagic disease. Vaccine 19, 4536–4543 (2001).
Schneider, M. C. et al. Potential force of infection of human rabies transmitted by vampire bats in the Amazonian region of Brazil. Am. J. Trop. Med. Hyg. 55, 680–684 (1996).
Belotto, A., Leanes, L. F., Schneider, M. C., Tamayp, H. & Correa, E. Overview of rabies in the Americas. Virus Res. 111, 5–12 (2005).
Benavides, J. A., Paniagua, E. R., Hampson, K., Valderrama, W. & Streicker, D. G. Quantifying the burden of vampire bat rabies in Peruvian livestock. PLoS Negl. Trop. Dis. 11, e0006105 (2017).
Schneider, M. C. et al. Rabies transmitted by vampire bats to humans: an emerging zoonotic disease in Latin America? Rev. Panam. Salud Públ. 25, 260–269 (2009).
Linhart, S. B., FloresCrespo, R. & Mitchell, G. C. Control of vampire bats by topical application of an anticoagulant, chlorophacinone. Bol. OSP 6, 31–38 (1972).
Fornes, A. et al. Control of bovine rabies through vampire bat control. J. Wildl. Dis. 10, 310–316 (1974).
Streicker, D. G. et al. Ecological and anthropogenic drivers of rabies exposure in vampire bats: implications for transmission and control. Proc. R. Soc. B Biol. Sci. 279, 3384–3392 (2012).
Blackwood, J. C., Streicker, D. G., Altizer, S. & Rohani, P. Resolving the roles of immunity, pathogenesis, and immigration for rabies persistence in vampire bats. Proc. Natl Acad. Sci. USA 110, 20837–20842 (2013).
Donnelly, C. A. et al. Impact of localized badger culling on tuberculosis incidence in British cattle. Nature 426, 834–837 (2003).
Aguilar-Setién, A. et al. Experimental rabies infection and oral vaccination in vampire bats (Desmodus rotundus). Vaccine 16, 1122–1126 (1998).
Aguilar-Setién, A. et al. Vaccination of vampire bats using recombinant vaccinia-rabies virus. J. Wildl. Dis. 38, 539–544 (2002).
Stading, B. et al. Protection of bats (Eptesicus fuscus) against rabies following topical or oronasal exposure to a recombinant raccoon poxvirus vaccine. PLoS Negl. Trop. Dis. 11, e0005958 (2017).
Almeida, M., Martorelli, L., Aires, C., Barros, R. & Massad, E. Vaccinating the vampire bat Desmodus rotundus against rabies. Virus Res. 137, 275–277 (2008).
Cagnacci, F., Massei, G., Coats, J., DeLeeuw, A. & Cowan, D. P. Long-lasting systemic bait markers for Eurasian badgers. J. Wildl. Dis. 42, 892–896 (2006).
Fry, T. L., Atwood, T. & Dunbar, M. R. Evaluation of rhodamine B as a biomarker for raccoons. Hum. Wildl. Interact. 4, 275–282 (2010).
Fernandez, J. R. R. & Rocke, T. E. Use of rhodamine B as a biomarker for oral plague vaccination of prairie dogs. J. Wildl. Dis. 47, 765–768 (2011).
Clay, C. A., Lehmer, E. M., Previtali, A., St Jeor, S. & Dearing, M. D. Contact heterogeneity in deer mice: implications for Sin Nombre virus transmission. Proc. R. Soc. B Biol. Sci. 276, 1305–1312 (2009).
Hoyt, J. R. et al. Cryptic connections illuminate pathogen transmission within community networks. Nature 563, 710–713 (2018).
Hampson, K. et al. Transmission dynamics and prospects for the elimination of canine rabies. PLoS Biol. 7, e1000053 (2009).
Amengual, B., Bourhy, H., López-Roíg, M. & Serra-Cobo, J. Temporal dynamics of European bat lyssavirus type 1 and survival of Myotis myotis bats in natural colonies. PLoS ONE 2, 0000566 (2007).
Streicker, D. G. et al. Host–pathogen evolutionary signatures reveal dynamics and future invasions of vampire bat rabies. Proc. Natl Acad. Sci. USA 113, 10926–10931 (2016).
Benavides, J. A., Valderrama, W. & Streicker, D. G. Spatial expansions and travelling waves of rabies in vampire bats. Proc. R. Soc. Lond. B Biol. Sci. 283, 20160328 (2016).
Choisy, M. & Rohani, P. Harvesting can increase severity of wildlife disease epidemics. Proc. R. Soc. B Biol. Sci. 273, 2025–2034 (2006).
Gomes, M. N., Uieda, W. & Latorre, M. Influence of sex differences in the same colony for chemical control of vampire Desmodus rotundus (Phyllostomidae) populations in the state of Sao Paulo, Brazil. Pesqui. Vet. Bras. 26, 38–43 (2006).
Wilkinson, G. S. Social grooming in the common vampire bat, Desmodus rotundus. Anim. Behav. 34, 1880–1889 (1986).
Carter, G. & Leffer, L. Social grooming in bats: are vampire bats exceptional? PLoS ONE 10, e0138430 (2015).
Bergner, L. M. et al. Using noninvasive metagenomics to characterize viral communities from wildlife. Mol. Ecol. Res. 19, 128–143 (2019).
Thompson, R. D., Elias, D. J. & Mitchell, G. C. Effects of vampire bat control on bovine milk production. J. Wildl. Manage. 41, 736–739 (1977).
Hardy, C. et al. Biological control of vertebrate pests using virally vectored immunocontraception. J. Reprod. Immunol. 71, 102–111 (2006).
Beyer, H. L. et al. The implications of metapopulation dynamics on the design of vaccination campaigns. Vaccine 30, 1014–1022 (2012).
Stading, B. R. et al. Infectivity of attenuated poxvirus vaccine vectors and immunogenicity of a raccoonpox vectored rabies vaccine in the Brazilian free-tailed bat (Tadarida brasiliensis). Vaccine 34, 5352–5358 (2016).
Langwig, K. E. et al. Resistance in persisting bat populations after white-nose syndrome invasion. Phil. Trans. R. Soc. B Biol. Sci. 372, 20160044 (2017).
Plowright, R. K. et al. Urban habituation, ecological connectivity and epidemic dampening: the emergence of Hendra virus from flying foxes (Pteropus spp.). Proc. R. Soc. B Biol. Sci. 278, 3703–3712 (2011).
Hayman, D. T. Biannual birth pulses allow filoviruses to persist in bat populations. Proc. R. Soc. B Biol. Sci. 282, 20142591 (2015).
Rocke, T. E. et al. Virally-vectored vaccine candidates against white-nose syndrome induce anti-fungal immune response in little brown bats (Myotis lucifugus). Sci. Rep. 9, 6788 (2019).
Marzi, A. et al. Cytomegalovirus-based vaccine expressing Ebola virus glycoprotein protects nonhuman primates from Ebola virus infection. Sci. Rep. 6, 21674 (2016).
Pallister, J. et al. A recombinant Hendra virus G glycoprotein-based subunit vaccine protects ferrets from lethal Hendra virus challenge. Vaccine 29, 5623–5630 (2011).
Olival, K. & Hayman, D. Filoviruses in bats: current knowledge and future directions. Viruses 6, 1759–1788 (2014).
Schnabel, Z. E. The estimation of total fish population of a lake. Am. Math. Monthly 45, 348–352 (1938).
Anthony, E. L. P. in Ecological and Behavioral Methods for the Study of Bats (ed. Kunz, T. H.) 47–58 (Smithsonian Institution Press, 1988).
Constantine, D. G., Tierkel, E. S., Kleckner, M. D. & Hawkins, D. M. Rabies in New Mexico cavern bats. Public Health Rep. 83, 303–316 (1968).
Delpietro, H., Russo, R., Carter, G., Lord, R. & Delpietro, G. Reproductive seasonality, sex ratio and philopatry in Argentina’s common vampire bats. R. Soc. Open Sci. 4, 160959 (2017).
Stading, B. Development of a Novel Rabies Mosaic Antigen and the Use of Attenuated Poxviruses as Vaccine Vectors in Bats (University of Wisconsin–Madison, 2015).
Acknowledgements
K.M.B. was supported by NIH award F32AI134016, and computational resources were provided by NIH award U01GM110712. D.G.S. was supported by a Sir Henry Dale Fellowship that was jointly funded by the Wellcome Trust and Royal Society (102507/Z/13/Z). Additional funding was provided via a Challenge Grant from the Royal Society to D.G.S., T.E.R., J.E.O., C.S. and N.F. (CH160097). The authors are grateful to D. Walsh, M. Viana and the Streicker Group for comments on earlier versions of this manuscript. Any use of trade, product or firm names does not imply endorsement by the US Government.
Author information
Authors and Affiliations
Contributions
D.G.S., T.E.R. and J.E.O. conceived and designed the experiments. R.C.A., C.T. and J.E.C. performed the experiments. K.M.B. and D.G.S. analysed the data. T.E.R., J.E.O., W.V., C.S. and N.F. contributed materials and/or analysis tools. K.M.B. and D.G.S. wrote the first draft of the paper. All authors contributed revisions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9, Tables 1–3, methods and results.
Supplementary Software 1
R script to carry out least-squares estimation of RB transfer rates.
Supplementary Software 2
R script to carry out mathematical modelling.
Source data
Source Data Fig. 1
RB marking and detection histories for wild bats in the field study.
Source Data Fig. 2
Ultraviolet powder marking and detection histories for wild bats in the field study.
Rights and permissions
About this article
Cite this article
Bakker, K.M., Rocke, T.E., Osorio, J.E. et al. Fluorescent biomarkers demonstrate prospects for spreadable vaccines to control disease transmission in wild bats. Nat Ecol Evol 3, 1697–1704 (2019). https://doi.org/10.1038/s41559-019-1032-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41559-019-1032-x
This article is cited by
-
Rabies in a postpandemic world: resilient reservoirs, redoubtable riposte, recurrent roadblocks, and resolute recidivism
Animal Diseases (2023)
-
A review of the diet of the common vampire bat (Desmodus rotundus) in the context of anthropogenic change
Mammalian Biology (2023)
-
Safety and security concerns regarding transmissible vaccines
Nature Ecology & Evolution (2021)
-
Knowledge gaps about rabies transmission from vampire bats to humans
Nature Ecology & Evolution (2020)
-
Epidemiology and biology of a herpesvirus in rabies endemic vampire bat populations
Nature Communications (2020)