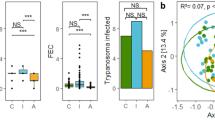
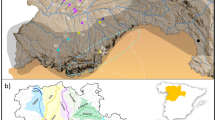
Abstract
Anthropogenic land-use change is an important driver of global biodiversity loss and threatens public health through biological interactions. Understanding these landscape–ecological effects at local scales will help achieve the United Nations Sustainable Development Goals by balancing urbanization, biodiversity and the spread of infectious diseases. Here, we address this knowledge gap by analysing a 43-year-long monthly dataset (1980–2022) of synanthropic rodents in Central China during intensive land-use change. We observed a notable increase in the mean patch size, coinciding with a substantial change in rodent community composition and a marked decline in rodent diversity; eight of the nine local rodent species experienced near-extirpation. Our analysis reveals that these irregular species replacements can be attributed to the effect of land consolidation on species competition among rodents, favouring striped field mice, a critical reservoir host of Hantaan virus (HTNV). Consequently, land consolidation has facilitated the proliferation of striped field mice and increased the prevalence of HTNV among them. This study highlights the importance of considering both direct and indirect effects of anthropogenic activities in the management of biodiversity and public health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information. Raw data are not publicly available and are protected due to confidentiality agreements, which were used under license for the current study but are available upon reasonable request to the corresponding author and with permission from the data provider (H.T.). The request will be responded to within 2 weeks.
Code availability
Code files are available via GitHub at https://github.com/huaiyutian/Hantaan-virus.
References
Wiens, J. A., Stenseth, N. C., Vanhorne, B. & Ims, R. A. Ecological mechanisms and landscape ecology. Oikos 66, 369–380 (1993).
Global Indicator Framework for the Sustainable Development Goals and targets of the 2030 Agenda for Sustainable Development (United Nations, 2020).
Seto, K. C. & Pandey, B. Urban land use: central to building a sustainable future. One Earth 1, 168–170 (2019).
Foley, J. A. et al. Global consequences of land use. Science 309, 570–574 (2005).
Shochat, E., Warren, P. S., Faeth, S. H., McIntyre, N. E. & Hope, D. From patterns to emerging processes in mechanistic urban ecology. Trends Ecol. Evol. 21, 186–191 (2006).
Faust, C. L. et al. Pathogen spillover during land conversion. Ecol. Lett. 21, 471–483 (2018).
Gibb, R. et al. Zoonotic host diversity increases in human-dominated ecosystems. Nature 584, 398–402 (2020).
Keesing, F. & Ostfeld, R. S. Impacts of biodiversity and biodiversity loss on zoonotic diseases. Proc. Natl Acad. Sci. USA 118, e2023540118 (2021).
Keesing, F. et al. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652 (2010).
Reynolds, J. D. in Macroecology Life Histories and Extinction Risk (ed. Blackburn, T. M.) 195–217 (Blackwell Publishing, 2003).
Ostfeld, R. S. & Keesing, F. Species that can make us ill thrive in human habitats. Nature 584, 346–347 (2020).
Essl, F. et al. Distribution patterns, range size and niche breadth of Austrian endemic plants. Biol. Conserv. 142, 2547–2558 (2009).
Kotiaho, J. S., Kaitala, V., Komonen, A. & Päivinen, J. Predicting the risk of extinction from shared ecological characteristics. Proc. Natl Acad. Sci. USA 102, 1963–1967 (2005).
Saupe, E. E. et al. Niche breadth and geographic range size as determinants of species survival on geological time scales. Glob. Ecol. Biogeogr. 24, 1159–1169 (2015).
Shultz, S., Bradbury, R. B., Evans, K. L., Gregory, R. D. & Blackburn, T. M. Brain size and resource specialization predict long-term population trends in British birds. Proc. R. Soc. B. 272, 2305–2311 (2005).
Walker, J. S. Resource use and rarity among frugivorous birds in a tropical rain forest on Sulawesi. Biol. Conserv. 130, 60–69 (2006).
White, R. L. & Bennett, P. M. Elevational distribution and extinction risk in birds. PLoS ONE 10, e0121849 (2015).
Liang, C. et al. Taxonomic, phylogenetic and functional homogenization of bird communities due to land use change. Biol. Conserv. 236, 37–43 (2019).
Colléony, A. & Shwartz, A. When the winners are the losers: invasive alien bird species outcompete the native winners in the biotic homogenization process. Biol. Conserv. 241, 108314 (2020).
Safi, K. & Kerth, G. A comparative analysis of specialization and extinction risk in temperate-zone bats. Conserv. Biol. 18, 1293–1303 (2004).
Boyles, J. G. & Storm, J. J. The perils of picky eating: dietary breadth is related to extinction risk in insectivorous bats. PLoS ONE 2, e672 (2007).
McKinney, M. L. & Lockwood, J. L. Biotic homogenization: a few winners replacing many losers in the next mass extinction. Trends Ecol. Evol. 14, 450–453 (1999).
Olden, J. D. Biotic homogenization: a new research agenda for conservation biogeography. J. Biogeogr. 33, 2027–2039 (2006).
Olden, J. D. & Rooney, T. P. On defining and quantifying biotic homogenization. Glob. Ecol. Biogeogr. 15, 113–120 (2006).
Clavel, J., Julliard, R. & Devictor, V. Worldwide decline of specialist species: toward a global functional homogenization? Front. Ecol. Environ. 9, 222–228 (2011).
Li, D. et al. Changes in taxonomic and phylogenetic diversity in the Anthropocene. Proc. R. Soc. B. 287, 20200777 (2020).
Jones, K. E. et al. Global trends in emerging infectious diseases. Nature 451, 990–993 (2008).
Keesing, F., Holt, R. D. & Ostfeld, R. S. Effects of species diversity on disease risk. Ecol. Lett. 9, 485–498 (2006).
Hansen, M. C. et al. High-resolution global maps of 21st-century forest cover change. Science 342, 850–853 (2013).
Ceballos, G. et al. Accelerated modern human-induced species losses: entering the sixth mass extinction. Sci. Adv. 1, e1400253 (2015).
Haddad, N. M. et al. Habitat fragmentation and its lasting impact on Earth’s ecosystems. Sci. Adv. 1, e1500052 (2015).
Ewers, R. M. et al. Logging cuts the functional importance of invertebrates in tropical rainforest. Nat. Commun. 6, 6836 (2015).
Newbold, T. et al. Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50 (2015).
The Sustainable Development Goals Report 2022 (UN DESA, 2022).
Fahrig, L. Effects of habitat fragmentation on biodiversity. Annu. Rev. Ecol. Evol. Syst. 34, 487–515 (2003).
Ewers, R. M. & Didham, R. K. Confounding factors in the detection of species responses to habitat fragmentation. Biol. Rev. Camb. Philos. Soc. 81, 117–142 (2006).
Zhou, Y., Li, Y. & Xu, C. Land consolidation and rural revitalization in China: mechanisms and paths. Land Use Policy 91, 104379 (2020).
Tian, H. Y. et al. Urbanization prolongs hantavirus epidemics in cities. Proc. Natl Acad. Sci. USA 115, 4707–4712 (2018).
Lin, Y., Han, G., Zhao, M. & Chang, S. X. Spatial vegetation patterns as early signs of desertification: a case study of a desert steppe in Inner Mongolia, China. Landsc. Ecol. 25, 1519–1527 (2010).
Uchida, K. & Ushimaru, A. Biodiversity declines due to abandonment and intensification of agricultural lands: patterns and mechanisms. Ecol. Monogr. 84, 637–658 (2014).
Maskell, L. C. et al. Exploring relationships between land use intensity, habitat heterogeneity and biodiversity to identify and monitor areas of high nature value farming. Biol. Conserv. 231, 30–38 (2019).
Denac, K. & Kmecl, P. Land consolidation negatively affects farmland bird diversity and conservation value. J. Nat. Conserv. 59, 125934 (2021).
McGarigal, K. & Cushman, S. A. Comparative evaluation of experimental approaches to the study of habitat fragmentation effects. Ecol. Appl. 12, 335–345 (2002).
Tian, H. et al. Interannual cycles of Hantaan virus outbreaks at the human–animal interface in Central China are controlled by temperature and rainfall. Proc. Natl Acad. Sci. USA 114, 8041–8046 (2017).
Fang, L. Z. et al. Reservoir host expansion of hantavirus, China. Emerg. Infect. Dis. 21, 170–171 (2015).
Jost, L. Entropy and diversity. Oikos 113, 363–375 (2006).
Pielou, E. C. Shannon’s formula as a measure of specific diversity: its use and misuse. Am. Nat. 100, 463–465 (1966).
Liu, Y., Yang, R. & Li, Y. Potential of land consolidation of hollowed villages under different urbanization scenarios in China. J. Geogr. Sci. 23, 503–512 (2013).
Thakkar, J.J. in Structural Equation Modelling (ed. Kacprzyk, J.) 91–99 (Springer, 2020).
Sugihara, G. et al. Detecting causality in complex ecosystems. Science 338, 496–500 (2012).
Gasparrini, A. Modeling exposure–lag–response associations with distributed lag non‐linear models. Stat. Med. 33, 881–899 (2014).
Hofbauer, J. & Sigmund, K. Evolutionary Games and Population Dynamics (Cambridge Univ. Press, 1998).
Shilova, S. A. & Tchabovsky, A. V. Population response of rodents to control with rodenticides. Curr. Zool. 55, 81–91 (2009).
Koelle, K. & Pascual, M. Disentangling extrinsic from intrinsic factors in disease dynamics: a nonlinear time series approach with an application to cholera. Am. Nat. 163, 901–913 (2004).
Koelle, K., Rodó, X., Pascual, M., Yunus, M. & Mostafa, G. Refractory periods and climate forcing in cholera dynamics. Nature 436, 696–700 (2005).
Benincà, E. et al. Chaos in a long-term experiment with a plankton community. Nature 451, 822–825 (2008).
Benincà, E., Ballantine, B., Ellner, S. P. & Huisman, J. Species fluctuations sustained by a cyclic succession at the edge of chaos. Proc. Natl Acad. Sci. USA 112, 6389–6394 (2015).
Gravinatti, M. L., Barbosa, C. M., Soares, R. M. & Gregori, F. Synanthropic rodents as virus reservoirs and transmitters. Rev. Soc. Bras. Med. Trop. 53, e20190486 (2020).
Sluydts, V., Davis, S., Mercelis, S. & Leirs, H. Comparison of multimammate mouse (Mastomys natalensis) demography in monoculture and mosaic agricultural habitat: implications for pest management. Crop Prot. 28, 647–654 (2009).
Mulungu, L. & Lopa, H. Comparative study of population dynamics and breeding patterns of Mastomys natalensis in system rice intensification and conventional rice production in irrigated rice ecosystems in Tanzania. J. Rice Res. 4, 161 (2016).
Li, J., Liu, Q., Yang, T. & Wang, C. A three year monitoring program of countrywide commensal rodent infestation in some towns and rural areas of China. Chin. J. Vec. Biol. Contr. 4, 276–281 (1988).
Zhang, M., Wang, Y., Li, B., Guo, C. & Chen, A. Effects of chemical rodent control on rodent community structure in the Yangtze River Basin. Acta Ecol. Sin. 2, 320–329 (2003).
Loreau, M. et al. Do not downplay biodiversity loss. Nature 601, E27–E28 (2022).
Dammhahn, M., Mazza, V., Schirmer, A., Göttsche, C. & Eccard, J. A. Of city and village mice: behavioural adjustments of striped field mice to urban environments. Sci. Rep. 10, 13056 (2020).
Tulis, F. et al. Expansion of the Striped field mouse (Apodemus agrarius) in the south-western Slovakia during 2010–2015. Folia Oecol. 43, 753 (2016).
Shochat, E., Lerman, S. B., Katti, M. & Lewis, D. B. Linking optimal foraging behavior to bird community structure in an urban-desert landscape: field experiments with artificial food patches. Am. Nat. 164, 232–243 (2004).
Petren, K. & Case, T. J. An experimental demonstration of exploitation competition in an ongoing invasion. Ecology 77, 118–132 (1996).
Dizney, L. J. & Ruedas, L. A. Increased host species diversity and decreased prevalence of Sin Nombre virus. Emerg. Infect. Dis. 15, 1012 (2009).
Dearing, M. D., Clay, C., Lehmer, E. & Dizney, L. The roles of community diversity and contact rates on pathogen prevalence. J. Mammal. 96, 29–36 (2015).
Prist, P. R. et al. Landscape, environmental and social predictors of Hantavirus risk in São Paulo, Brazil. PLoS ONE 11, e0163459 (2016).
Prist, P. R., D´ Andrea, P. S. & Metzger, J. P. Landscape, climate and hantavirus cardiopulmonary syndrome outbreaks. Ecohealth 14, 614–629 (2017).
Shaanxi Statistical Yearbook 2010 (China Statistics Press, 2010).
Crecente, R., Alvarez, C. & Fra, U. Economic, social and environmental impact of land consolidation in Galicia. Land Use Policy 19, 135–147 (2002).
Pašakarnis, G. & Maliene, V. Towards sustainable rural development in Central and Eastern Europe: applying land consolidation. Land Use Policy 27, 545–549 (2010).
Rosseel, Y. lavaan: an R package for structural equation modeling. J. Stat. Softw. 48, 1–36 (2012).
Park, J., Smith, C., Sugihara, G. & Deyle, E. rEDM: Empirical dynamic modeling (‘EDM’). R package version 1.9.1 https://CRAN.R-project.org/package=rEDM (2021).
R Core Team A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021).
Kareiva, P. Finding and losing host plants by Phyllotreta: patch size and surrounding habitat. Ecology 66, 1809–1816 (1985).
Bach, C. E. Effects of host plant patch size on herbivore density: underlying mechanisms. Ecology 69, 1103–1117 (1988).
Bender, D. J., Contreras, T. A. & Fahrig, L. Habitat loss and population decline: a meta‐analysis of the patch size effect. Ecology 79, 517–533 (1998).
Acknowledgements
We thank the hundreds of CDC staff and local health workers in Shaanxi province who collected successive data from 1980 to 2022. We are also deeply grateful to P. Zheng for her valuable contributions to this paper. This study was supported by the Fundamental Research Funds for the Central Universities (2233300001); National Key Research and Development Program of China (2022YFC2303803); scientific and technological innovation 2030—major project of new generation artificial intelligence (2021ZD0111201); National Natural Science Foundation of China (82073616, 82204160); research on Key Technologies of Plague Prevention and Control in Inner Mongolia Autonomous Region (2021ZD0006); BNU-FGS Global Environmental Change Program (no. 2023-GC-ZYTS-11); and key research projects of Beijing Natural Science Foundation-Haidian District Joint Fund (L232014). J.R., O.G.P. and C.F. were funded by NSF/BBSRC project ‘Integrating metaviromics with epidemiological dynamics: understanding virus transmission in the Anthropocene’ (BB/Y006879/1). The funders had no role in study design, data collection and analysis, the decision to publish, or in preparation of the paper.
Author information
Authors and Affiliations
Contributions
H.T. conceived the study. H.T., O.G.P., A.P.D. and N.C.S. jointly supervised this work. P.Y., T.Z., J.Q. and J.W. collected the statistical data. S.P., Y.W., Y. Liang and Y.C. conducted the analyses. P.Y., J.R., Z.L., Q.L., C.S., G.D., C.L.F., J.Q., J.W., S.L., T.Z., C.M., N.B., B.C., R.Y., O.G.P., A.P.D., Y.X., Y. Li and N.C.S. edited the paper. H.T., S.P., Y.C., Y. Li, Y.W., Y.T. and J.R. wrote the paper. All authors read and approved the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Greg Adler, Jinbao Liao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Overview of study system, Supplementary Figs. 1–16 and Tables 1–4.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pei, S., Yu, P., Raghwani, J. et al. Anthropogenic land consolidation intensifies zoonotic host diversity loss and disease transmission in human habitats. Nat Ecol Evol 9, 99–110 (2025). https://doi.org/10.1038/s41559-024-02570-x
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41559-024-02570-x
This article is cited by
-
Species diversity links land consolidation to rodent disease
Nature Ecology & Evolution (2024)