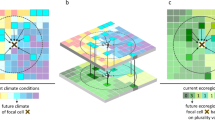
Abstract
Human activities have transformed many wild and semiwild ecosystems into novel states without historical precedent. Without knowing the current distribution of what drives the emergence of such novelty, predicting future ecosystem states and informing conservation and restoration policies remain difficult. Here we construct global maps of three key drivers generating novel conditions—climate change, defaunation and floristic disruption—and summarize them to a measure of total novelty exposure. We show that the terrestrial biosphere is widely exposed to novel conditions, with 58% of the total area exposed to high levels of total novelty. All climatic regions and biomes are exposed to substantial levels of novelty. Relative contributions of individual drivers vary between climatic regions, with climate changes and defaunation the largest contributors globally. Protected areas and key biodiversity areas, whether formally protected or not, have similar exposure, with high total novelty experienced in 58% of cells inside protected areas and 56% inside key biodiversity areas. Our results highlight the importance of investigating ecosystem and biodiversity responses to rising ecological novelty for informing actions towards biosphere stewardship.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All data used in the study are from publicly available sources. Climate and palaeoclimate data are both available from https://chelsa-climate.org/ (refs. 43,75) and ice sheet data are available from https://www.atmosp.physics.utoronto.ca/~peltier/data.php. Layers used for building the defaunation data are available from https://megapast2future.github.io/PHYLACINE_1.2/. The exact GBIF data used for generating the alien species occurrence data in this study are available from https://doi.org/10.15468/dl.6aebcd (ref. 80); the authors note that a more recent download of GBIF data may result in slightly different results. The country-level checklists from GRIIS are available via Zenodo at https://zenodo.org/records/6348164 (ref. 83). The BII was accessed directly from refs. 55,95. The WDPA can be accessed from https://www.protectedplanet.net and KBAs can be requested from keybiodiversityareas.org. The three novelty process layers generated in this study, as well as the total novelty exposure layer, are available via Zenodo at https://doi.org/10.5281/zenodo.14677611 (ref. 96).
Code availability
All analysis code is available via GitHub at https://github.com/KerrMatt/NoveltyMapping and in the Supplementary Code.
References
Newbold, T. et al. Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50 (2015).
Gordon, J. D., Fagan, B., Milner, N. & Thomas, C. D. Floristic diversity and its relationships with human land use varied regionally during the Holocene. Nat. Ecol. Evol. 8, 1459–1471 (2024).
Svenning, J.-C., Kerr, M. R., Mungi, N. A., Ordonez, A. & Riede, F. Defining the Anthropocene as a geological epoch captures human impacts’ triphasic nature to empower science and action. One Earth https://doi.org/10.1016/j.oneear.2024.08.004 (2024).
Burke, K. D. et al. Pliocene and Eocene provide best analogs for near-future climates. Proc. Natl Acad. Sci. USA 115, 13288–13293 (2018).
Technical Summary. Climate Change 2021: The Physical Science Basis (eds Masson-Delmotte, V. et al.) 35–144 (Cambridge Univ. Press, 2023).
Barnosky, A. D. et al. Approaching a state shift in Earth’s biosphere. Nature 486, 52–58 (2012).
Ellis, E. C. et al. People have shaped most of terrestrial nature for at least 12,000 years. Proc. Natl Acad. Sci. USA 118, e2023483118 (2021).
Clement, S. & Standish, R. J. Novel ecosystems: governance and conservation in the age of the Anthropocene. J. Environ. Manag. 208, 36–45 (2018).
Arias, S. The abandonment of the ideal of wilderness: rewilding as the consequence of the Anthropocene metaphysics on restoration ecology. Anthrop. Rev. https://doi.org/10.1177/20530196241270671 (2024).
Hobbs, R. J. et al. Novel ecosystems: theoretical and management aspects of the new ecological world order. Glob. Ecol. Biogeogr. 15, 1–7 (2006).
Kerr, M. R., Ordonez, A., Riede, F. & Svenning, J.-C. A biogeographic–macroecological perspective on the rising novelty of the biosphere in the Anthropocene. J. Biogeogr. 51, 575–587 (2024).
Ordonez, A. & Gill, J. L. Unravelling the functional and phylogenetic dimensions of novel ecosystem assemblages. Phil. Trans. R. Soc. B 379, 20230324 (2024).
Morse, N. B. et al. Novel ecosystems in the Anthropocene: a revision of the novel ecosystem concept for pragmatic applications. Ecol. Soc. 19, 12 (2014).
Evers, C. R. et al. The ecosystem services and biodiversity of novel ecosystems: a literature review. Glob. Ecol. Conserv. 13, e00362 (2018).
Dornelas, M. et al. Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299 (2014).
O’Dea, A. et al. Defining variation in pre-human ecosystems can guide conservation: an example from a Caribbean coral reef. Sci. Rep. 10, 2922 (2020).
Williams, J. W. & Jackson, S. T. Novel climates, no-analog communities, and ecological surprises. Front. Ecol. Environ. 5, 475–482 (2007).
Staples, T. L., Kiessling, W. & Pandolfi, J. M. Emergence patterns of locally novel plant communities driven by past climate change and modern anthropogenic impacts. Ecol. Lett. 25, 1497–1509 (2022).
Williams, J. W., Ordonez, A. & Svenning, J.-C. A unifying framework for studying and managing climate-driven rates of ecological change. Nat. Ecol. Evol. 5, 17–26 (2021).
Albano, P. G. et al. The dawn of the tropical Atlantic invasion into the Mediterranean Sea. Proc. Natl Acad. Sci. USA 121, e2320687121 (2024).
Svenning, J.-C. & Sandel, B. Disequilibrium vegetation dynamics under future climate change. Am. J. Bot. 100, 1266–1286 (2013).
Williams, J. W., Jackson, S. T. & Kutzbach, J. E. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl Acad. Sci. USA 104, 5738–5742 (2007).
Ordonez, A., Riede, F., Normand, S. & Svenning, J.-C. Towards a novel biosphere in 2300: rapid and extensive global and biome-wide climatic novelty in the Anthropocene. Phil. Trans. R. Soc. B 379, 20230022 (2024).
Davis, M., Faurby, S. & Svenning, J.-C. Mammal diversity will take millions of years to recover from the current biodiversity crisis. Proc. Natl Acad. Sci. USA 115, 11262–11267 (2018).
Radeloff, V. C. et al. The rise of novelty in ecosystems. Ecol. Appl. 25, 2051–2068 (2015).
Berti, E. & Svenning, J.-C. Megafauna extinctions have reduced biotic connectivity worldwide. Glob. Ecol. Biogeogr. 29, 2131–2142 (2020).
Svenning, J.-C. et al. The late-Quaternary megafauna extinctions: patterns, causes, ecological consequences and implications for ecosystem management in the Anthropocene. Cambridge Prisms: Extinction 2, e5 (2024).
Fehr, V., Buitenwerf, R. & Svenning, J.-C. Non-native palms (Arecaceae) as generators of novel ecosystems: a global assessment. Divers. Distrib. 26, 1523–1538 (2020).
Walentowitz, A. et al. Long-term trajectories of non-native vegetation on islands globally. Ecol. Lett. 26, 729–741 (2023).
Jaureguiberry, P. et al. The direct drivers of recent global anthropogenic biodiversity loss. Sci. Adv. 8, eabm9982 (2022).
Sheffer, E. A review of the development of Mediterranean pine–oak ecosystems after land abandonment and afforestation: are they novel ecosystems? Ann. For. Sci. 69, 429–443 (2012).
Keith, S. A., Newton, A. C., Herbert, R. J. H., Morecroft, M. D. & Bealey, C. E. Non-analogous community formation in response to climate change. J. Nat. Conserv. 17, 228–235 (2009).
Volpe, J. P. et al. Bionovelty and ecological restoration. Restor. Ecol. 32, e14152 (2024).
Hobbs, R. J., Higgs, E. & Harris, J. A. Novel ecosystems: implications for conservation and restoration. Trends Ecol. Evol. 24, 599–605 (2009).
Trew, B. T., Lees, A. C., Edwards, D. P., Early, R. & Maclean, I. M. D. Identifying climate-smart tropical Key Biodiversity Areas for protection in response to widespread temperature novelty. Conserv. Lett. 17, e13050 (2024).
McGeoch, M. A., Clarke, D. A., Mungi, N. A. & Ordonez, A. A nature-positive future with biological invasions: theory, decision support and research needs. Phil. Trans. R. Soc. B 379, 20230014 (2024).
Lundgren, E. J. et al. Introduced herbivores restore Late Pleistocene ecological functions. Proc. Natl Acad. Sci. USA 117, 7871–7878 (2020).
Kharouba, H. M. Shifting the paradigm: the role of introduced plants in the resiliency of terrestrial ecosystems to climate change. Glob. Change Biol. 30, e17319 (2024).
Gill, J. L. et al. A 2.5-million-year perspective on coarse-filter strategies for conserving nature’s stage. Conserv. Biol. 29, 640–648 (2015).
Pandolfi, J. M., Staples, T. L. & Kiessling, W. Increased extinction in the emergence of novel ecological communities. Science 370, 220–222 (2020).
Ordonez, A., Williams, J. W. & Svenning, J.-C. Mapping climatic mechanisms likely to favour the emergence of novel communities. Nat. Clim. Change 6, 1104–1109 (2016).
Hobbs, R. J., Higgs, E. S. & Hall, C. M. in Novel Ecosystems (eds Hobbs, R. J. et al.) 58–60 (Wiley, 2013).
Karger, D. N., Nobis, M. P., Normand, S., Graham, C. & Zimmermann, N. CHELSA-TraCE21k—high-resolution (1 km) downscaled transient temperature and precipitation data since the Last Glacial Maximum. Climate 19, 439–456 (2023).
Williams, J. J. & Newbold, T. Local climatic changes affect biodiversity responses to land use: a review. Divers. Distrib. 26, 76–92 (2020).
Faurby, S. et al. PHYLACINE 1.2: the phylogenetic atlas of mammal macroecology. Ecology 99, 2626 (2018).
Bar-On, Y. M., Phillips, R. & Milo, R. The biomass distribution on Earth. Proc. Natl Acad. Sci. USA 115, 6506–6511 (2018).
Burke, K. D. et al. Differing climatic mechanisms control transient and accumulated vegetation novelty in Europe and eastern North America. Phil. Trans. R. Soc. B 374, 20190218 (2019).
Fastovich, D., Radeloff, V. C., Zuckerberg, B. & Williams, J. W. Legacies of millennial-scale climate oscillations in contemporary biodiversity in eastern North America. Phil. Trans. R. Soc. B 379, 20230012 (2024).
Dirzo, R. et al. Defaunation in the Anthropocene. Science 345, 401–406 (2014).
Galetti, M. et al. Ecological and evolutionary legacy of megafauna extinctions. Biol. Rev. 93, 845–862 (2018).
Mungi, N. A., Jhala, Y. V., Qureshi, Q., le Roux, E. & Svenning, J.-C. Megaherbivores provide biotic resistance against alien plant dominance. Nat. Ecol. Evol. 7, 1645–1653 (2023).
Carter, B. E. & Alroy, J. Energy use of modern terrestrial large mammal communities mirrors Late Pleistocene megafaunal extinctions. Front. Biogeogr. 16.2, e62724 (2024).
Schittko, C. et al. A multidimensional framework for measuring biotic novelty: how novel is a community? Glob. Change Biol. 26, 4401–4417 (2020).
Roy, H. E. et al. Curbing the major and growing threats from invasive alien species is urgent and achievable. Nat. Ecol. Evol. 8, 1216–1223 (2024).
Newbold, T. et al. Has land use pushed terrestrial biodiversity beyond the planetary boundary? A global assessment. Science 353, 288–291 (2016).
Scholes, R. J. & Biggs, R. A biodiversity intactness index. Nature 434, 45–49 (2005).
Davoli, M. et al. Megafauna diversity and functional declines in Europe from the Last Interglacial to the present. Glob. Ecol. Biogeogr. 33, 34–47 (2024).
Neugarten, R. A. et al. Mapping the planet’s critical areas for biodiversity and nature’s contributions to people. Nat. Commun. 15, 261 (2024).
Langhammer, P. F., Mittermeier, R. A., Plumptre, A. J., Waliczky, Z. & Sechrest, W. Key Biodiversity Areas (CEMEX, 2021).
The Relationship between Key Biodiversity Areas (KBAs) and Protected Areas (Key Biodiversity Areas Partnership, 2017).
Protected Planet: The World Database on Protected Areas (WDPA) and World Database on Other Effective Area-based Conservation Measures (WD-OECM) [Online] (UNEP-WCMC & IUCN, 2024).
Bingham, H. C. et al. Sixty years of tracking conservation progress using the World Database on Protected Areas. Nat. Ecol. Evol. 3, 737–743 (2019).
Butchart, S. H. M. et al. Protecting important sites for biodiversity contributes to meeting global conservation targets. PLoS ONE 7, e32529 (2012).
Duncanson, L. et al. The effectiveness of global protected areas for climate change mitigation. Nat. Commun. 14, 2908 (2023).
Garcia, R. A., Cabeza, M., Rahbek, C. & Araújo, M. B. Multiple dimensions of climate change and their implications for biodiversity. Science 344, 1247579 (2014).
Xie, Y., Wang, X. & Silander, J. A. Deciduous forest responses to temperature, precipitation, and drought imply complex climate change impacts. Proc. Natl Acad. Sci. USA 112, 13585–13590 (2015).
Fløjgaard, C., Pedersen, P. B. M., Sandom, C. J., Svenning, J.-C. & Ejrnæs, R. Exploring a natural baseline for large-herbivore biomass in ecological restoration. J. Appl. Ecol. 59, 18–24 (2022).
Trepel, J. et al. Zoogeochemistry of a protected area: driven by anthropogenic impacts and animal behavior. Conserv. Sci. Pract. 6, e13107 (2024).
Ganz, T. R. et al. Cougars, wolves, and humans drive a dynamic landscape of fear for elk. Ecology 105, e4255 (2024).
Svenning, J.-C., Buitenwerf, R. & Le Roux, E. Trophic rewilding as a restoration approach under emerging novel biosphere conditions. Curr. Biol. 34, R435–R451 (2024).
Stein, A., Gerstner, K. & Kreft, H. Environmental heterogeneity as a universal driver of species richness across taxa, biomes and spatial scales. Ecol. Lett. 17, 866–880 (2014).
Prober, S. M. et al. Climate-adjusted provenancing: a strategy for climate-resilient ecological restoration. Front. Ecol. Evol. 3, 65 (2015).
Delavaux, C. S. et al. Native diversity buffers against severity of non-native tree invasions. Nature 621, 773–781 (2023).
Fricke, E. C., Ordonez, A., Rogers, H. S. & Svenning, J.-C. The effects of defaunation on plants’ capacity to track climate change. Science 375, 210–214 (2022).
Karger, D. N., Nobis, M. P., Normand, S., Graham, C. H. & Zimmermann, N. E. CHELSA-TraCE21k: Downscaled Transient Temperature and Precipitation Data Since the Last Glacial Maximum. EnviDat https://doi.org/10.16904/envidat.211 (2020).
Argus, D. F., Peltier, W. R., Drummond, R. & Moore, A. W. The Antarctica component of postglacial rebound model ICE-6G_C (VM5a) based on GPS positioning, exposure age dating of ice thicknesses, and relative sea level histories. Geophys. J. Int. 198, 537–563 (2014).
Peltier, W. R., Argus, D. F. & Drummond, R. Space geodesy constrains ice age terminal deglaciation: the global ICE-6G_C (VM5a) model. J. Geophy. Res. 120, 450–487 (2015).
Richard Peltier, W., Argus, D. F. & Drummond, R. Comment on “An assessment of the ICE-6G_C (VM5a) glacial isostatic sdjustment model” by Purcell et al. J. Geophys. Res. 123, 2019–2028 (2018).
Faurby, S. et al. MegaPast2Future/PHYLACINE_1.2: PHYLACINE Version 1.2.1. Zenodo https://doi.org/10.5281/zenodo.3690867 (2020).
GBIF occurrence download. GBIF https://doi.org/10.15468/DL.6AEBCD (2023).
Chamberlain, S. et al. rgbif: interface to the Global Biodiversity Information Facility API. R package v.3.7.8 (2025).
Zizka, A. et al. CoordinateCleaner: standardized cleaning of occurrence records from biological collection databases. Methods Ecol. Evol. 10, 744–751 (2019).
Pagad, S., Genovesi, P., Carnevali, L., Schigel, D. & McGeoch, M. A. Introducing the global register of introduced and invasive species. Sci. Data 5, 170202 (2018).
Pagad, S. et al. Country compendium of the global register of introduced and invasive species. Sci. Data 9, 391 (2022).
Beck, H. E. et al. High-resolution (1 km) Köppen–Geiger maps for 1901–2099 based on constrained CMIP6 projections. Sci. Data 10, 724 (2023).
Allen, J. R. M. et al. Global vegetation patterns of the past 140,000 years. J. Biogeogr. 47, 2073–2090 (2020).
Fischer, J.-C., Walentowitz, A. & Beierkuhnlein, C. The biome inventory—standardizing global biogeographical land units. Glob. Ecol. Biogeogr. 31, 2172–2183 (2022).
The World Database of Key Biodiversity Areas (KBA, accessed 20 February 2024); www.keybiodiversityareas.org
Popovic, G. et al. Four principles for improved statistical ecology. Methods Ecol. Evol. 15, 266–281 (2024).
Sullivan, G. M. & Feinn, R. Using effect size—or why the P value is not enough. J. Grad. Med. Educ. 4, 279–282 (2012).
Hedges, L. V. Distribution theory for Glass’s estimator of effect size and related estimators. J. Educ. Stat. 6, 107–128 (1981).
Lovakov, A. & Agadullina, E. R. Empirically derived guidelines for effect size interpretation in social psychology. Eur. J. Soc. Psychol. 51, 485–504 (2021).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2023).
Crameri, F., Shephard, G. E. & Heron, P. J. The misuse of colour in science communication. Nat. Commun. 11, 5444 (2020).
Newbold, T. et al. Map of Biodiversity Intactness Index (from Global map of the Biodiversity Intactness Index, from Newbold et al. (2016) Science) [dataset resource]. Natural History Museum https://data.nhm.ac.uk/dataset/global-map-of-the-biodiversity-intactness-index-from-newbold-et-al-2016-science/resource/8531b4dc-bd44-4586-8216-47b3b8d60e85 (2016).
Kerr, M. R. Processed novelty layers for climate, defaunation, floristic disruption, and total novelty exposure, from Kerr et al 2025 ‘Widespread ecological novelty across the terrestrial biosphere’ [dataset]. Zenodo https://doi.org/10.5281/zenodo.14677612 (2025).
Acknowledgements
We thank Danish National Research Foundation for economic support via Center for Ecological Dynamics in a Novel Biosphere (ECONOVO; grant number DNRF173 to J.-C.S.). We thank O. A. Baines and C. W. Davison for advice on spatial analysis and R. Ø. Pedersen for advice on generating the defaunation metrics.
Author information
Authors and Affiliations
Contributions
M.R.K., A.O., F.R. and J.-C.S. conceptualized the study, formulated the approaches used and led discussions. M.R.K. led investigation, analysed data and led interpretation with input from all authors. M.R.K. wrote the first draft. Review and editing were carried out by all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Jenny McGuire, Anna Walentowitz and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Total novelty exposure across the terrestrial biosphere.
The scaled total novelty exposure when all three drivers of novelty are given equal weighting. The legend shows the frequency of cells at each novelty level, with the vertical solid line marking the global median (0.35). Map is shown in an equal area Mollweide projection, cells are 10x10 km.
Extended Data Fig. 2 Key drivers of novelty across the terrestrial biosphere.
Locally novel conditions are caused by combinations of the three drivers, with cells coloured based on the relative contributions of each driver, as shown in the inset ternary plot. Map is shown in an equal area Mollweide projection, cells are 10×10 km.
Extended Data Fig. 3 The biosphere is highly exposed to multiple novelty drivers.
Novelty is shown in each map as the scaled exposure when both individual metrics are given equal weighting. The legend shows the frequency of cells at each novelty level, with the vertical solid line marking the global median. a, Climate change novelty, composed of temperature and precipitation metrics; b, defaunation novelty, composed of richness and body mass changes of mammal communities; c, floristic disruption novelty, composed of the change in intactness and the proportion of alien species in plant communities. Maps in each panel are shown in an equal area Mollweide projection. Cells in each map are 10×10 km.
Extended Data Fig. 4 Drivers of novelty are unevenly distributed between cells globally and within broad climate regions.
Ternary plots show the relative contribution of each driver, with darker colours representing a larger proportion of analysed cells for that set of contributions. Each axis is divided into 50 bins. Data is shown both within broad climate regions and for all cells (‘Global’, bottom right).
Extended Data Fig. 5 Today’s biosphere is highly novel, even when accounting for spatial bias in biodiversity data.
Maps show different ways of accounting for spatial bias in the biodiversity data which feeds into our measurement of alien species distribution. a, cells lacking biodiversity data are excluded from the analysis entirely, leaving only cells with a value for all six metrics shown. b, the alien species metric is excluded from the analysis and novelty is calculated using only the five other metrics. The legend shows the frequency of cells at each novelty level, with the vertical solid line marking the global median. Maps in each panel are shown in an equal area Mollweide projection. Cells in each map are 10×10 km.
Extended Data Fig. 6 Biomes are slightly unevenly exposed to novel conditions.
a, scaled total novelty exposure, equally weighted between climate change, defaunation and floristic disruption. b, the contribution of each individual novelty process to the total novelty varies between biomes. Biomes are taken from Fischer et al87 abbreviated as follows (with the number of 10×10 km cells in each biome in parentheses); TrEF, Tropical evergreen forest (n = 1,115,644); TrRF, Tropical raingreen forest (n = 994,819); SAV, Savanna (n = 406,931); TrGL, Tropical grassland (n = 328,622); WTW, Warm temperate woodland (n = 184,160); DES, Desert (n = 1,107,836); TBF, Temperate broadleaf evergreen forest (n = 562,133); SDES, Semi-desert (n = 350,957); TeSL, Temperate shrubland (n = 971,411); TeNF, Temperate needleleaf evergreen forest (n = 31,691); STE, Steppe (n = 134,741); TePL, Temperate Parkland (n = 266,201); TeSF, Temperate summergreen forest (n = 266,201); TeMF, Temperate mixed forest (n = 276,547); BPL, Boreal Parkland (n = 476,718); TUN, Tundra (n = 62,559); BSBF, Boreal summergreen broadleaf forest (n = 237,111); BEF, Boreal evergreen needleleaf forest (n = 653,171); BSNF, Boreal summergreen needleleaf forest (32,594); STUN, Shrub tundra (n = 445,889); BWL, Boreal woodland (291,822). Novelty drivers in b are left-to-right ordered as in the legend In the box plots the central line represents the median, the upper and lower box limits represent the first and third quartiles respectively. Whiskers extend to 1.5 times the interquartile range. Pairwise effect sizes are available as Supplemental Tables 5-8.
Extended Data Fig. 7 Drivers of novelty evenly affect areas of differing protection status.
Data shown are for each individual novelty driver – climate novelty (top), defaunation novelty (middle) and floristic disruption (bottom). Cells are divided into broad climatic regions; the global results are shown to the right of the vertical black line. Colours indicate the protection category of each cell as either a ‘protected area’ or not inside a protected area, with data from outside protected areas generated from 1000 iterations of random, equal area draws from the same climate region for each protected area In the box plots the central line represents the median, the upper and lower box limits represent the first and third quartiles respectively. Whiskers extend to 1.5 times the interquartile range. Numbers above each pair are the effect size (Hedges G) for that comparison. All pairwise comparisons are significant (in each case p < 0.0001 in a two-sided, unpaired t-test with Welch’s correction).
Extended Data Fig. 8 Drivers of novelty evenly affect areas of differing biodiversity status.
Data shown are for each individual novelty driver – climate novelty (top), defaunation novelty (middle) and floristic disruption (bottom). Cells are divided into broad climatic regions; the global results are shown to the right of the vertical black line. Colours indicate the biodiversity category of each cell as either a “Key Biodiversity Area” or not inside a Key Biodiversity Area, with data from outside Key Biodiversity Areas generated from 1000 iterations of random, equal area draws from the same climate region for each Key Biodiversity Area. In the box plots the central line represents the median, the upper and lower box limits represent the first and third quartiles respectively. Whiskers extend to 1.5 times the interquartile range. Numbers above each pair are the effect size (Hedges G) for that comparison. All pairwise comparisons are significant (in each case p < 0.0001 in a two-sided, unpaired t-test with Welch’s correction).
Supplementary information
Supplementary Information
Supplementary Tables 1–8.
Supplementary Code
R files: (1) outlines the calculation of the novelty layers from raw data; (2) outlines the analysis in the paper; and (3) outlines the figure creation.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kerr, M.R., Ordonez, A., Riede, F. et al. Widespread ecological novelty across the terrestrial biosphere. Nat Ecol Evol 9, 589–598 (2025). https://doi.org/10.1038/s41559-025-02662-2
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41559-025-02662-2
This article is cited by
-
Predator-prey temporal niche partitioning under human disturbance: a meta-analysis
Nature Communications (2026)
-
Ecological processes shaping Antarctic terrestrial biodiversity change
Nature Reviews Biodiversity (2026)
-
Ecological novelty is the new norm on our planet
Nature Ecology & Evolution (2025)
-
Sound science
Nature Ecology & Evolution (2025)
-
Consider the costs of connectivity when creating a well-connected Earth
Nature Reviews Biodiversity (2025)