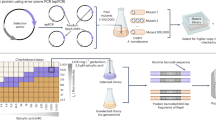
Abstract
Multicopy plasmids are widespread in nature and compose a common strategy for spreading beneficial traits across microbes. However, the role of plasmids in supporting the evolution of encoded genes remains underexplored due to challenges in experimentally manipulating key parameters such as plasmid copy number and mutation rate. Here we developed a strategy for controlling copy number in the plasmid-based Saccharomyces cerevisiae continuous evolution system, OrthoRep, and used our resulting capabilities to investigate the evolution of a conditionally essential gene under varying copy number and mutation rate conditions. Our results show that low copy number facilitated the faster enrichment of beneficial alleles whereas high copy number promoted robustness through the maintenance of allelic diversity. High copy number also slowed the removal of deleterious mutations and increased the fraction of non-functional alleles that could hitchhike during evolution. This study highlights the nuanced relationships between plasmid copy number, mutation rate and evolutionary outcomes, providing insights into the adaptive dynamics of genes encoded on multicopy plasmids and nominating OrthoRep as a versatile tool for studying plasmid evolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Raw sequencing data can be found on the Sequence Read Archive under accession number PRJNA1236365. Comprehensive mutational data can be found in Supplementary Table 3.
Code availability
Custom code is described as pseudocode in the methods and Python scripts can be found via Zenodo at https://doi.org/10.5281/zenodo.15604699 (ref. 41) and MAPLE nanopore analysis via GitHub at https://github.com/gordonrix/maple.
References
Rodríguez-Beltrán, J., DelaFuente, J., León-Sampedro, R., MacLean, R. C. & San Millán, Á. Beyond horizontal gene transfer: the role of plasmids in bacterial evolution. Nat. Rev. Microbiol. 19, 347–359 (2021).
Fukuhara, H. Linear DNA plasmids of yeasts. FEMS Microbiol. Lett. 131, 1–9 (1995).
Handa, H. Linear plasmids in plant mitochondria: peaceful coexistences or malicious invasions? Mitochondrion 8, 15–25 (2008).
Pilla, G. & Tang, C. M. Going around in circles: virulence plasmids in enteric pathogens. Nat. Rev. Microbiol. 16, 484–495 (2018).
Brinkmann, H., Göker, M., Koblížek, M., Wagner-Döbler, I. & Petersen, J. Horizontal operon transfer, plasmids, and the evolution of photosynthesis in Rhodobacteraceae. ISME J. 12, 1994–2010 (2018).
Manzano-Marı́n, A. et al. Serial horizontal transfer of vitamin-biosynthetic genes enables the establishment of new nutritional symbionts in aphids’ di-symbiotic systems. ISME J. 14, 259–273 (2020).
Moraïs, S. et al. Plasmid-encoded toxin defence mediates mutualistic microbial interactions. Nat. Microbiol. 9, 108–119 (2024).
San Millan, A. & MacLean, R. C. Fitness costs of plasmids: a limit to plasmid transmission. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.mtbp-0016-2017 (2017).
Rouches, M. V., Xu, Y., Cortes, L. B. G. & Lambert, G. A plasmid system with tunable copy number. Nat. Commun. 13, 3908 (2022).
Ilhan, J. et al. Segregational drift and the interplay between plasmid copy number and evolvability. Mol. Biol. Evol. 36, 472–486 (2019).
Rodriguez-Beltran, J. et al. Multicopy plasmids allow bacteria to escape from fitness trade-offs during evolutionary innovation. Nat. Ecol. Evol. 2, 873–881 (2018).
San Millan, A., Escudero, J. A., Gifford, D. R., Mazel, D. & MacLean, R. C. Multicopy plasmids potentiate the evolution of antibiotic resistance in bacteria. Nat. Ecol. Evol. 1, 0010 (2016).
Frank, S. A. Multiplicity of infection and the evolution of hybrid incompatibility in segmented viruses. Heredity 87, 522–529 (2001).
Santer, M., Kupczok, A., Dagan, T. & Uecker, H. Fixation dynamics of beneficial alleles in prokaryotic polyploid chromosomes and plasmids. Genetics 222, iyac121 (2022).
Dewan, I. & Uecker, H. A mathematician’s guide to plasmids: an introduction to plasmid biology for modellers. Microbiology 169, 001362 (2023).
Santer, M. & Uecker, H. Evolutionary rescue and drug resistance on multicopy plasmids. Genetics 215, 847–868 (2020).
Mei, H., Arbeithuber, B., Cremona, M. A., DeGiorgio, M. & Nekrutenko, A. A high-resolution view of adaptive event dynamics in a plasmid. Genome Biol. Evol. 11, 3022–3034 (2019).
Ravikumar, A., Arrieta, A. & Liu, C. C. An orthogonal DNA replication system in yeast. Nat. Chem. Biol. 10, 175–177 (2014).
Ravikumar, A., Arzumanyan, G. A., Obadi, M. K. A., Javanpour, A. A. & Liu, C. C. Scalable, continuous evolution of genes at mutation rates above genomic error thresholds. Cell 175, 1946–1957.e13 (2018).
Rix, G. et al. Continuous evolution of user-defined genes at 1 million times the genomic mutation rate. Science 386, eadm9073 (2024).
Wu, Z., Liu, C., Zhang, Z., Zheng, R. & Zheng, Y. Amidase as a versatile tool in amide-bond cleavage: from molecular features to biotechnological applications. Biotechnol. Adv. 43, 107574 (2020).
Patrick, W. M., Quandt, E. M., Swartzlander, D. B. & Matsumura, I. Multicopy suppression underpins metabolic evolvability. Mol. Biol. Evol. 24, 2716–2722 (2007).
Keren, L. et al. Promoters maintain their relative activity levels under different growth conditions. Mol. Syst. Biol. 9, 701 (2013).
Ravasio, R. et al. A minimal scenario for the origin of non-equilibrium order. Preprint at https://arxiv.org/abs/2405.10911 (2025).
Solis-Escalante, D. et al. amdsym, a new dominant recyclable marker cassette for Saccharomyces cerevisiae. FEMS Yeast Res. 13, 126–139 (2013).
Diercks, C. S. et al. An orthogonal T7 replisome for continuous hypermutation and accelerated evolution in E. coli. Preprint at bioRxiv https://doi.org/10.1101/2024.07.25.605042 (2024).
Tian, R. et al. Establishing a synthetic orthogonal replication system enables accelerated evolution in E. coli. Science 383, 421–426 (2024).
Molina, R. S. et al. In vivo hypermutation and continuous evolution. Nat. Rev. Methods Primers 2, 36 (2022).
Stiffler, M. A., Hekstra, D. R. & Ranganathan, R. Evolvability as a function of purifying selection in TEM-1 β-lactamase. Cell 160, 882–892 (2015).
Wrenbeck, E. E., Azouz, L. R. & Whitehead, T. A. Single-mutation fitness landscapes for an enzyme on multiple substrates reveal specificity is globally encoded. Nat. Commun. 8, 15695 (2017).
Sarkisyan, K. S. et al. Local fitness landscape of the green fluorescent protein. Nature 533, 397–401 (2016).
Rodríguez-Beltrán, J. et al. Genetic dominance governs the evolution and spread of mobile genetic elements in bacteria. Proc. Natl Acad. Sci. USA 117, 15755–15762 (2020).
Duffy, S. Why are RNA virus mutation rates so damn high? PLoS Biol. 16, e3000003 (2018).
Holmes, E. C. Error thresholds and the constraints to RNA virus evolution. Trends Microbiol. 11, 543–546 (2003).
Sýkora, M. et al. Transcription apparatus of the yeast virus-like elements: architecture, function, and evolutionary origin. PLoS Pathog. 14, e1007377 (2018).
Brachmann, C. B. et al. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14, 115–132 (1998).
Javanpour, A. A. & Liu, C. C. Genetic compatibility and extensibility of orthogonal replication. ACS Synth. Biol. 8, 1249–1256 (2019).
Gietz, R. D. & Schiestl, R. H. Frozen competent yeast cells that can be transformed with high efficiency using the LIAC/SS carrier DNA/peg method. Nat. Protoc. 2, 1–4 (2007).
Georgieva, B. & Rothstein, R. in Methods in Enzymology (eds Guthrie, C. & Fink, G. R.) 278–289 (Academic Press, 2002).
Blount, B. A., Driessen, M. R. & Ellis, T. GC PREPS: fast and easy extraction of stable yeast genomic DNA. Sci. Rep. 6, 26863 (2016).
Pisera, O. copy_fitness_analysis: v1 copy number evolution assessment. Zenodo https://doi.org/10.5281/zenodo.15604699 (2025).
Acknowledgements
This work was funded by NIH R35GM136297 to C.C.L. A.‘O.’P. is supported by a Paul and Daisy Soros Fellowship for New Americans.
Author information
Authors and Affiliations
Contributions
A.‘O.’P. and C.C.L. conceived the project ideas. A.‘O.’P. designed and carried out all experiments, collected and processed all data and analysed all data with input from C.C.L. A.‘O.’P. and C.C.L. wrote the paper.
Corresponding author
Ethics declarations
Competing interests
C.C.L. is a co-founder of K2 Therapeutics, which uses OrthoRep in antibody engineering and evolution. The other authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Fabienne Benz, Alvaro San Millan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8.
Supplementary Tables
Supplementary Tables 1–5.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Pisera, A.‘., Liu, C.C. The role of plasmid copy number and mutation rate in evolutionary outcomes. Nat Ecol Evol 9, 1694–1704 (2025). https://doi.org/10.1038/s41559-025-02792-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41559-025-02792-7