Abstract
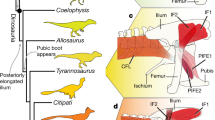
An enlarged sternum with a prominent keel is a central feature of the flight apparatus of modern birds. However, sterna of near-bird dinosaurs (Pennaraptora) and early avialans are either substantially different from those of living birds or absent altogether, raising questions about how specialized sternal structures evolved in birds and how they are related to function. This remains poorly understood because of the fragmentary nature of the fossil record, and the challenges in inferring form and function from crushed fossils. We use ancestral character estimations to trace sternal trait acquisition through the bird stem group, and multivariate phylogenetic regressions to analyse relationships between sternum morphology, body mass and flight capabilities. We find that sternum evolution was episodic: basal members of Pennaraptora had proportionally small sterna, which became larger and more craniocaudally elongated in Avialae. This enlargement precedes the appearance of a midline ridge, a possible precursor of the sternal keel, in Pygostylia. Sternum size increased again in crownward Ornithuromorpha, alongside a fully formed sternal keel and enlarged caudal projections, both critical areas of flight muscle attachment. Sternal experimentation in relation to flight characteristics occurs several times throughout Pennaraptora, including within Paraves and Enantiornithes, indicating that powered flight may have evolved several times before proliferating in crown-group birds.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Data generated and analysed in this study are available via Dryad at https://doi.org/10.5061/dryad.jsxksn0ng (ref. 135).
Code availability
R code for analyses performed here are available via Dryad at https://doi.org/10.5061/dryad.jsxksn0ng (ref. 135).
References
Hou, L., Martin, L. D., Zhou, Z. & Feduccia, A. Early adaptive radiation of birds: evidence from fossils from northeastern China. Science 274, 1164–1167 (1996).
Chiappe, L. M. The first 85 million years of avian evolution. Nature 378, 349–355 (1995).
Owen, R. On the Anatomy of Vertebrates: Birds and Mammals (Longmans, Green and Company, 1866).
O’Connor, J. K. et al. Evolution and functional significance of derived sternal ossification patterns in ornithothoracine birds. J. Evol. Biol. 28, 1550–1567 (2015).
Mayr, G. Pectoral girdle morphology of Mesozoic birds and the evolution of the avian supracoracoideus muscle. J. Ornithol. 158, 859–867 (2017).
Lowi-Merri, T., Benson, R. B. J., Claramunt, S. & Evans, D. C. The relationship between sternum variation and mode of locomotion in birds. BMC Biol. 19, 165 (2021).
Nopcsa, B. F. Ideas on the origin of flight. Proc. Zool. Soc. Lond. 77, 223–236 (1907).
Ostrom, J. H. Archaeopteryx and the origin of flight. Q. Rev. Biol. 49, 27–47 (1974).
Padian, K. Cross-testing adaptive hypotheses: phylogenetic analysis and the origin of bird flight. Am. Zool. 41, 598–607 (2001).
Zhou, Z. & Zhang, F. Mesozoic birds of China—a synoptic review. Front. Biol. China 2, 1–14 (2007).
Lindsay, B. On the avian sternum. Proc. Zool. Soc. Lond. 53, 684–716 (1885).
Zheng, X., Wang, X., O’Connor, J. & Zhou, Z. Insight into the early evolution of the avian sternum from juvenile enantiornithines. Nat. Commun. 3, 1116–1118 (2012).
Lull, R. S. Volant adaptation in vertebrates. Am. Nat. 40, 537–566 (1906).
Ridpath, M. The Tasmanian native hen, Tribonyx mortierii. III. Ecology. CSIRO Wildl. Res. 17, 91–118 (1972).
Ostrom, J. H. Archaeopteryx and the origin of birds. Biol. J. Linn. Soc. 8, 91–182 (1976).
Heimerdinger, M. A. & Ames, P. L. Variation in the sternal notches of suboscine passeriform birds. Postilla 105, 1–44 (1967).
Feduccia, A. The Origin and Evolution of Birds (Yale Univ. Press, 1996).
Wang, M. & Zhou, Z. in The Biology of the Avian Respiratory System: Evolution, Development, Structure and Function (ed. Maina, J.) 1–26 (Springer, 2017).
Burgers, P. & Chiappe, L. M. The wing of Archaeopteryx as a primary thrust generator. Nature 399, 60–62 (1999).
Dececchi, T. A., Larsson, H. C. E. & Habib, M. B. The wings before the bird: an evaluation of flapping-based locomotory hypotheses in bird antecedents. PeerJ 4, e2159 (2016).
Heers, A. M., Baier, D. B., Jackson, B. E. & Dial, K. P. Flapping before flight: high resolution, three-dimensional skeletal kinematics of wings and legs during avian development. PLoS ONE 11, e0153446 (2016).
Burnham, D. A. Archaeopteryx—a re-evaluation suggesting an arboreal habitat and an intermediate stage in trees down origin of flight. Neues Jb. Geol. Paläontol. Abh. 245, 33–44 (2007).
Dial, K. P., Randall, R. J. & Dial, T. R. What use is half a wing in the ecology and evolution of birds? Bioscience 56, 437–445 (2006).
Nudds, R. L. & Dyke, G. J. Forelimb posture in dinosaurs and the evolution of the avian flapping flight-stroke. Evolution 63, 994–1002 (2009).
Senter, P. Scapular orientation in the theropods and basal birds, and the origin of flapping flight. Acta Palaeontol. Pol. 51, 305–313 (2006).
Voeten, D. F. A. E. et al. Wing bone geometry reveals active flight in Archaeopteryx. Nat. Commun. 9, 923 (2018).
Serrano, F. J. & Chiappe, L. M. Aerodynamic modelling of a Cretaceous bird reveals thermal soaring capabilities during early avian evolution. J. R. Soc. Interface 14, 20170182 (2017).
Pei, R. et al. Potential for powered flight neared by most close avialan relatives, but few crossed its thresholds. Curr. Biol. 30, 4033–4046 (2020).
Dial, K. P. Wing-assisted incline running and the evolution of flight. Science 299, 402–404 (2003).
Jackson, B. E., Tobalske, B. W. & Dial, K. P. The broad range of contractile behaviour of the avian pectoralis: functional and evolutionary implications. J. Exp. Biol. 214, 2354–2361 (2011).
Marsh, O. C. Odontornithes: A Monograph of the Extinct Toothed Birds of North America (G.P.O, 1880).
Zheng, X. et al. On the absence of sternal elements in Anchiornis (Paraves) and Sapeornis (Aves) and the complex early evolution of the avian sternum. Proc. Natl Acad. Sci. USA 111, 13900–13905 (2014).
Pittman, M. et al. Preserved soft anatomy confirms shoulder-powered upstroke of early theropod flyers, reveals enhanced early pygostylian upstroke, and explains early sternum loss. Proc. Natl Acad. Sci. USA 119, e2205476119 (2022).
Wang, X. et al. Basal paravian functional anatomy illuminated by high-detail body outline. Nat. Commun. 8, 14576 (2017).
Zheng, X. et al. Structure and possible ventilatory function of unusual, expanded sternal ribs in the Early Cretaceous bird Jeholornis. Cretac. Res. 116, 104597 (2020).
Hou, L.-H. et al. Confuciusornis sanctus, a new Late Jurassic sauriurine bird from China. Chinese Sci. Bull. 40, 1545–1551 (1995).
O’Connor, J. K., Zheng, X.-T., Hu, H., Wang, X.-L. & Zhou, Z.-H. The morphology of Chiappeavis magnapremaxillo (Pengornithidae: Enantiornithes) and a comparison of aerodynamic function in Early Cretaceous avian tail fans. Vertebr. Palasiat. 55, 41–58 (2017).
Wang, M., Oconnor, J. K. & Zhou, Z. A new robust enantiornithine bird from the Lower Cretaceous of China with scansorial adaptations. J. Vertebr. Paleontol. 34, 657–671 (2014).
O’Connor, J. K., Chiappe, L. M., Gao, C. & Zhao, B. Anatomy of the early cretaceous enantiornithine bird Rapaxavis pani. Acta Palaeontol. Pol. 56, 463–475 (2011).
Wu, Q., O’Connor, J. K., Wang, S. & Zhou, Z. Transformation of the pectoral girdle in pennaraptorans: critical steps in the formation of the modern avian shoulder joint. PeerJ 12, e16960 (2024).
Wang, S. et al. Digital restoration of the pectoral girdles of two Early Cretaceous birds, and implications for early flight evolution. eLife 11, e76086 (2022).
Heers, A. M., Varghese, S. L., Hatier, L. K. & Cabrera, J. J. Multiple functional solutions during flightless to flight-capable transitions. Front. Ecol. Evol. 8, 573411 (2021).
Lowi-Merri, T. M. et al. Reconstructing locomotor ecology of extinct avialans: a case study of Ichthyornis comparing sternum morphology and skeletal proportions. Proc. R. Soc. B 290, 20222020 (2023).
Xu, X. et al. Four-winged dinosaurs from China. Nature 421, 335–340 (2003).
Godfrey, S. J. & Currie, P. J. A in Feathered Dragons: Studies on the Transition from Dinosaurs to Birds (eds Currie, P. J. et al.) 144–149 (Indiana Univ. 2004).
Chiappe, L. M., Ji, S., Ji, Q. & Norell, M. A. Anatomy and Systematics of the Confuciusornithidae (Theropoda: Aves) from the Late Mesozoic of Northeastern China (American Museum of Natural History, 1999).
Martin, L. D., Zhou, Z., Hou, L. & Feduccia, A. Confuciusornis sanctus compared to Archaeopteryx lithographica. Naturwissenschaften 85, 286–289 (1998).
O’Connor, J. K., Zheng, X., Xiaoli, W., Zhang, X. & Zhou, Z. The gastral basket in basal birds and their close relatives: size and possible function. Vertebr. Palasiat. 53, 133–152 (2015).
Wang, M. & Zhou, Z. A morphological study of the first known piscivorous enantiornithine bird from the Early Cretaceous of China. J. Vertebr. Paleontol. 37, e1278702 (2017).
Hou, L., Chiappe, L. M., Zhang, F. & Chuong, C. M. New Early Cretaceous fossil from China documents a novel trophic specialization for Mesozoic birds. Naturwissenschaften 91, 22–25 (2004).
Chiappe, L. M. & Calvo, J. O. Neuquenornis volans, a new late Cretaceous bird (Enantiornithes: Avisauridae) from Patagonia, Argentina. J. Vertebr. Paleontol. 14, 230–246 (1994).
Wang, Y. M., O’Connor, J. K., Li, D. Q. & You, H. L. New information on postcranial skeleton of the Early Cretaceous Gansus yumenensis (Aves: Ornithuromorpha). Hist. Biol. 28, 666–679 (2016).
Zhou, S., Zhou, Z. & O’Connor, J. A new piscivorous ornithuromorph from the Jehol Biota. Hist. Biol. 26, 608–618 (2014).
Wang, M., Zhou, Z. & Zhou, S. A new basal ornithuromorph bird (Aves: Ornithothoraces) from the Early Cretaceous of China with implication for morphology of early Ornithuromorpha. Zool. J. Linn. Soc. 176, 207–223 (2016).
Wang, M., Zhou, Z. & Zhou, S. Corrigendum: Renaming of Bellulia Wang, Zhou & Zhou, 2016. Zool. J. Linn. Soc. 177, 695 (2016).
O’Connor, J. K., Wang, M., Zhou, S. & Zhou, Z. Osteohistology of the Lower Cretaceous Yixian formation ornithuromorph (Aves) Iteravis huchzermeyeri. Palaeontol. Electron. 18.2.35A, 1–11 (2015).
Lewis, P. O. A likelihood approach to estimating phylogeny from discrete morphological character data. Syst. Biol. 50, 913–925 (2001).
Bell, A. & Chiappe, L. M. The Hesperornithiformes: a review of the diversity, distribution, and ecology of the earliest diving birds. Diversity 14, 267 (2022).
Orkney, A., Bjarnason, A., Tronrud, B. C. & Benson, R. B. J. Patterns of skeletal integration in birds reveal that adaptation of element shapes enables coordinated evolution between anatomical modules. Nat. Ecol. Evol. 5, 1250–1258 (2021).
Zhang, Y.-G., Li, Z., Liu, D., Liu, Q.-G. & Fu, L. The diversity of morphology of the avian sternum and its relationship with flight ability. Sichuan J. Zool. 5, 677–686 (2011).
Yu, Y., Zhang, C. & Xu, X. Deep time diversity and the early radiations of birds. Proc. Natl Acad. Sci. USA 118, e2019865118 (2021).
Wang, M., Lloyd, G. T., Zhang, C., Zhou, Z. & Lloyd, G. T. The patterns and modes of the evolution of disparity in Mesozoic birds. Proc. R. Soc. B 288, 20203105 (2021).
Allen, V., Bates, K. T., Li, Z. & Hutchinson, J. R. Linking the evolution of body shape and locomotor biomechanics in bird-line archosaurs. Nature 497, 104–107 (2013).
Macaulay, S. et al. Decoupling body shape and mass distribution in birds and their dinosaurian ancestors. Nat. Commun. 14, 1575 (2023).
Olson, S. & Feduccia, A. Flight capability and the pectoral girdle of Archaeopteryx. Nature 278, 247–248 (1979).
Chamberlain, F. W. Atlas of Avian Anatomy: Osteology-Arthrology-Myology (Michigan State College Agricultural Experiment Station, 1943).
Baumel, J. J. & Witmer, L. M. in Handbook of Avian Anatomy: Nomina Anatomica Avium (eds Baumel, J. J. et al.) 45–131 (Nuttall Ornithological Club, 1993).
Bock, W. J. The furcula and the evolution of avian flight. Paleontol. J. 47, 1236–1244 (2013).
Meyers, R. A. & Stakebake, E. F. Anatomy and histochemistry of spread-wing posture in birds. 3. Immunohistochemistry of flight muscles and the ‘shoulder lock’ in albatrosses. J. Morphol. 263, 12–29 (2005).
Pennycuick, C. J. Animal Flight (Edward Arnold, 1972).
Zhou, Z. & Farlow, J. O. in New Perspectives on the Origin and Early Evolution of Birds: Proceedings of the International Symposium in Honor of John H. Ostrom (eds Gauthier, J. & Gall, L. F.) 237–254 (Peabody Museum of Natural History Yale Univ., 2001).
Rayner, J. M. V. in New Perspectives on the Origin and Early Evolution of Birds: Proceedings of the International Symposium in Honor of John H. Ostrom (eds Gauthier, J. A. & Gall, L. F.) 363–388 (Peabody Museum of Natural History Yale Univ., 2001).
Sy, M. Funktionell-anatomische Untersuchungen am Vogelflügel. J. Ornithol. 84, 199–296 (1936).
Brown, R. H. J. The flight of birds. Biol. Rev. 38, 460–489 (1963).
Pennycuick, C. J. Modelling the Flying Bird (Elsevier, 2008).
Chiappe, L. M. & Walker, C. A. in Mesozoic Birds: Above the Heads of Dinosaurs (eds Chiappe, L. M. & Witmer, L. M.) 240–267 (Univ. California Press, 2002).
Wong, M. & Carter, D. Mechanical stress and morphogenetic endochondral ossification of the sternum. J. Bone Joint Surg. Am. 70, 992–1000 (1988).
Falk, A. R., Kaye, T. G., Zhou, Z. & Burnham, D. A. Laser fluorescence illuminates the soft tissue and life habits of the early Cretaceous bird Confuciusornis. PLoS ONE 11, e0167284 (2016).
Feduccia, A. The Age of Birds (Harvard Univ. Press, 1980).
Burnham, D. A., Feduccia, A., Martin, L. D. & Falk, A. R. Tree climbing—a fundamental avian adaptation. J. Syst. Palaeontol. 9, 103–107 (2011).
Jasinoski, S. C., Russell, A. P. & Currie, P. J. An integrative phylogenetic and extrapolatory approach to the reconstruction of dromaeosaur (Theropoda: Eumaniraptora) shoulder musculature. Zool. J. Linn. Soc. 146, 301–344 (2006).
Walker, C. A. & Dyke, G. J. Euenantiornithine birds from the Late Cretaceous of El Brete (Argentina). Ir. J. Earth Sci. 27, 15–62 (2009).
Atterholt, J., Howard Hutchison, J. & O'Connor, J. K. The most complete enantiornithine from North America and a phylogenetic analysis of the Avisauridae. PeerJ 2018, e5910 (2018).
Happ, J., Elsler, A., Kriwet, J., Pfaff, C. & Bochenski, Z. M. Two passeriform birds (Aves: Passeriformes) from the Middle Miocene of Austria. PalZ 96, 313–321 (2022).
Chiappe, L. M., Serrano, F. J., Abramowicz, S. & Göhlich, U. B. Flight performance of the Early Cretaceous bird Confuciusornis sanctus: evidence from an exceptionally preserved fossil. Span. J. Palaeontol. 38, 101–122 (2023).
Xu, X. et al. A new feathered maniraptoran dinosaur fossil that fills a morphological gap in avian origin. Chin. Sci. Bull. 54, 430–435 (2009).
Gao, C. et al. A subadult specimen of the early Cretaceous bird Sapeornis chaoyangensis and a taxonomic reassessment of sapeornithids. J. Vertebr. Paleontol. 32, 1103–1112 (2012).
Kundrát, M., Nudds, J., Kear, B. P., Lü, J. & Ahlberg, P. The first specimen of Archaeopteryx from the Upper Jurassic Mörnsheim Formation of Germany. Hist. Biol. 31, 3–63 (2019).
Longrich, N. R., Vinther, J., Meng, Q., Li, Q. & Russell, A. P. Primitive wing feather arrangement in Archaeopteryx lithographica and Anchiornis huxleyi. Curr. Biol. 22, 2262–2267 (2012).
Lambertz, M. & Perry, S. F. Remarks on the evolution of the avian sternum, dinosaur gastralia, and their functional significance for the respiratory apparatus. Zool. Anz. 255, 80–84 (2015).
Xu, X. et al. An integrative approach to understanding bird origins. Science 346, 1253293 (2014).
Wang, Y. M., O’Connor, J. K., Li, D. Q. & You, H. L. Previously unrecognized ornithuromorph bird diversity in the Early Cretaceous Changma Basin, Gansu Province, Northwestern China. PLoS ONE 8, e77693 (2013).
Vazquez, R. J. The automating skeletal and muscular mechanisms of the avian wing (Aves). Zoomorphology 114, 59–71 (1994).
Clarke, J. A., Zhou, Z. & Zhang, F. Insight into the evolution of avian flight from a new clade of Early Cretaceous ornithurines from China and the morphology of Yixianornis grabaui. J. Anat. 208, 287–308 (2006).
Pittman, M. et al. Exceptional preservation and foot structure reveal ecological transitions and lifestyles of early theropod flyers. Nat. Commun. 13, 7684 (2022).
Bell, A. & Chiappe, L. M. Statistical approach for inferring ecology of Mesozoic birds. J. Syst. Palaeontol. 9, 119–133 (2011).
Brusatte, S. L., O’Connor, J. K. & Jarvis, E. D. The origin and diversification of birds. Curr. Biol. 25, R888–R898 (2015).
Pei, R., Li, Q., Meng, Q., Norell, M. A. & Gao, K. Q. New Specimens of Anchiornis huxleyi (Theropoda: Paraves) from the Late Jurassic of Northeastern China (American Museum of Natural History, 2017).
Zhou, Z. & Zhang, F. Anatomy of the primitive bird Sapeornis chaoyangensis from the Early Cretaceous of Liaoning, China. Can. J. Earth Sci. 40, 731–747 (2003).
Borchers, H. W. & Borchers, M. H. W. pracma. R package version 2.4.2. (2022).
Schneider, C. A., Rasband, W. S. & Eliceiri, K. W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 9, 671–675 (2012).
Anderson, J. F., Hall‐Martin, A. & Russell, D. A. Long‐bone circumference and weight in mammals, birds and dinosaurs. J. Zool. 207, 53–61 (1985).
Christiansen, P. & Fariña, R. A. Mass prediction in theropod dinosaurs. Hist. Biol. 16, 85–92 (2004).
Campbell, K. E. Jr & Marcus, L. in Papers in Avian Paleontology Honoring Pierce Brodkorb 395–412 (Natural History Museum of Los Angeles County, 1992).
Campione, N. E., Evans, D. C., Brown, C. M. & Carrano, M. T. Body mass estimation in non-avian bipeds using a theoretical conversion to quadruped stylopodial proportions. Methods Ecol. Evol. 5, 913–923 (2014).
Campione, N. E. Extrapolating body masses in large terrestrial vertebrates. Paleobiology 43, 693–699 (2017).
Campione, N. E. MASSTIMATE: body mass estimation equations for vertebrates. R package version 2.0 (2022).
Gingerich, P. D. Arithmetic or geometric normality of biological variation: an empirical test of theory. J. Theor. Biol. 204, 201–221 (2000).
Cooney, C. R. et al. Mega-evolutionary dynamics of the adaptive radiation of birds. Nature 542, 344–347 (2017).
Prum, R. O. et al. A comprehensive phylogeny of birds (Aves) using targeted next-generation DNA sequencing. Nature 526, 569–573 (2015).
Jetz, W., Thomas, G. H., Joy, J. B., Hartmann, K. & Mooers, A. O. The global diversity of birds in space and time. Nature 491, 444–448 (2012).
Bell, M. A. & Lloyd, G. T. strap. R package version 1.6-0 (2022).
del Hoyo, J., Elliott, A., Sargatal, J., Christie, D. A. & de Juana, E. (eds) Handbook of the Birds of the World Alive (Lynx Edicions, 2014).
Birds of the World (Cornell Laboratory of Ornithology, 2022).
Grafen, A. The phylogenetic regression. Philos. Trans. R. Soc. Lond. B https://doi.org/10.1098/rstb.1989.0106 (1989).
Orme, D. et al. caper. R package version 1.0.1 (2018).
Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Contr. 19, 716–723 (1974).
Sugiura, N. Further analysis of the data by Akaike’s information criterion and the finite corrections. Commun. Stat. Theory Method. 7, 13–26 (1978).
Choiniere, J. N. et al. Evolution of vision and hearing modalities in theropod dinosaurs. Science 613, 610–613 (2021).
Fabbri, M. et al. Subaqueous foraging among carnivorous dinosaurs. Nature 603, 852–857 (2022).
Felsenstein, J. Phylogenies and the comparative method. Am. Nat. 125, 1–15 (1985).
Goolsby, E. W. Rapid maximum likelihood ancestral state reconstruction of continuous characters: a rerooting-free algorithm. Ecol. Evol. 7, 2791–2797 (2017).
Revell, L. J. & Harmon, L. J. Phylogenetic Comparative Methods in R (Princeton Univ. Press, 2022).
Revell, L. J. phytools. R package version 1.5-1 (2023).
Bollback, J. P. SIMMAP: stochastic character mapping of discrete traits on phylogenies. BMC Bioinf. 7, 88 (2006).
Revell, L. J. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012).
Motani, R. & Schmitz, L. Phylogenetic versus functional signals in the evolution of form–function relationships in terrestrial vision. Evolution 65, 2245–2257 (2011).
Schmitz, L. & Motani, R. Nocturnality in dinosaurs inferred from scleral ring and orbit morphology. Science 332, 705–708 (2011).
Hermanson, G. et al. Cranial ecomorphology of turtles and neck retraction as a possible trigger of ecological diversification. Evolution 76, 2566–2586 (2022).
Navalón, G., Bjarnason, A., Griffiths, E. & Benson, R. B. J. Environmental signal in the evolutionary diversification of bird skeletons. Nature 611, 306–311 (2022).
Watanabe, J. Quantitative discrimination of flightlessness in fossil Anatidae from skeletal proportions. Auk 134, 672–695 (2017).
Stoessel, A., Kilbourne, B. M. & Fischer, M. S. Morphological integration versus ecological plasticity in the avian pelvic limb skeleton. J. Morphol. 274, 483–495 (2013).
Mosimann, J. E. Size allometry: size and shape variables with characterizations of the lognormal and generalized gamma distributions. J. Am. Stat. Assoc. 65, 930–945 (1970).
Adams, D. C. A method for assessing phylogenetic least squares models for shape and other high-dimensional multivariate data. Evolution 68, 2675–2688 (2014).
Lowi-Merri, T. M. et al. Data from: Enlargement of sternum traits facilitated the evolution of powered flight in birds. Dryad https://doi.org/10.5061/dryad.jsxksn0ng (2025).
Acknowledgements
We would like to thank A. Bailleul, M. Wang and Z. Zhonghe at the Institute of Vertebrate Palaeontology and Palaeoanthropology, as well as H. Gibbins, C. Coy and P. Currie at the University of Alberta Laboratory for Vertebrate Palaeontology for assistance and access to fossil specimens. J. Benito and D. Field (University of Cambridge) provided the reconstruction of Ichthyornis used in this study. Useful discussions, guidance and methodological assistance were provided by G. Funston, L. Mahler, J. Weir, D. McLennan and H. Larsson. This work was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) postgraduate scholarship (PGSD3-547147-2020) to T.M.L.-M., as well as NSERC Discovery Grants to D.C.E. (RGPIN-2018-06788) and S.C. (RGPIN-2018-06747). R.B. acknowledges support from the European Union’s Horizon 2020 research and innovation programme 2014–2018 under grant agreement no. 677774 (European Research Council starting grant: TEMPO). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
T.M.L.-M., R.B., S.C. and D.C.E. conceived and designed the project. R.B., H.H. and J.O. provided substantial data and verified data quality. T.M.L.-M. collected morphological and stratigraphic data, conducted all analyses and interpretations and wrote the initial paper draft. T.M.L.-M., R.B., H.H., J.O., S.C. and D.C.E. edited the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Michael Pittman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Discriminant analysis results.
Results from 100 iterations of the phylogenetic flexible discriminant analysis (pFDA) for the presence of forelimb propulsion ability in each of the fossil species, based on relative footprint area to body mass (N = 112 extant taxa; N = 28 fossil taxa). Blue values are from the pFDA that incorporates minimum body mass estimates, and orange values are maximum body mass estimates; corresponding dotted lines represent first quartile, median, and third quartile of estimates over 100 replicates of the analysis. X-axes represent probabilities for the presence of forelimb propulsion over 100 iterations of the analysis, and y-axes represent frequency of probabilities across 100 iterations. The red dotted line in the middle represents the 50% probability for flight presence, and the grey rectangle demarcates 0.33 to 0.67 probability, or the zone of uncertainty. Median probabilities (‘Mdn’) for flight presence are given for both body mass estimates. Values that are greater than 0.67 indicate that flight is likely present in the taxon, while less than 0.33 indicates that flight is likely absent; median probabilities in between fall in the uncertainty zone. Panels A-Z correspond with analyses performed for each fossil taxon.
Extended Data Fig. 2 Ancestral character estimation from results of discriminant analysis.
Ancestral character estimation of median probability of flight presence across the dinosaur-bird transition from the discriminant analysis based on relative sternum plate area using minimum body mass estimates (left) and maximum body mass estimates (right) (N = 28 fossil taxa). Darker colours indicate a likely presence of flight ability in the taxon, lighter colours indicate likely absence of flight ability. The extant portion of the tree was condensed into a single branch labelled ‘Neornithes’, setting the tip value at 0, which represents the relative value for the ancestor of the living bird crown group, Neornithes.
Supplementary information
Supplementary Information
Supplementary Results, Figs. 1–9 and Tables 1–10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lowi-Merri, T.M., Benson, R., Hu, H. et al. Enlargement of sternum traits facilitated the evolution of powered flight in birds. Nat Ecol Evol 9, 1705–1718 (2025). https://doi.org/10.1038/s41559-025-02795-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41559-025-02795-4