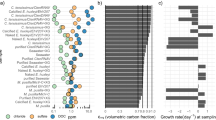
Abstract
More than 10 years following the onset of the sea star wasting disease (SSWD) epidemic, affecting over 20 asteroid species from Mexico to Alaska, the causative agent has been elusive. SSWD killed billions of the most susceptible species, sunflower sea stars (Pycnopodia helianthoides), initiating a trophic cascade involving unchecked urchin population growth and the widespread loss of kelp forests. Identifying the causative agent underpins the development of recovery strategies. Here we induced disease and subsequent mortality in exposure experiments using tissue extracts, coelomic fluid and effluent water from wasting sunflower sea stars, with no mortality in controls. Deep sequencing of diseased sea star coelomic fluid samples from experiments and field outbreaks revealed a dominant proportion of reads assigned to the bacterium Vibrio pectenicida. Fulfilling Koch’s postulates, V. pectenicida strain FHCF-3, cultured from the coelomic fluid of a diseased sunflower sea star, caused disease and mortality in exposed sunflower sea stars, demonstrating that it is a causative agent of SSWD. This discovery will enable recovery efforts for sea stars and the ecosystems affected by their decline by facilitating culture-based experimental research and broad-scale screening for pathogen presence and abundance in the laboratory and field.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Metatranscriptomic and 16S rRNA gene sequence datasets are archived in the NCBI Short Read Archive (BioProject no. PRJNA1195080). The whole-genome of V. pectenicida strain FHCF-3 is available from the NCBI GenBank Repository (accession no. JBLZMR000000000), with raw sequence reads archived in the NCBI Short Read Archive (BioProject no. PRJNA1232168). The complete 16S rRNA gene sequences of V. pectenicida strain FHCF-3 are deposited in the NCBI GenBank Repository (accessions PQ700178 and PQ763222–PQ763229). Source data are provided with this paper.
Code availability
Code generated in this study is available via Dryad at https://doi.org/10.5061/dryad.5mkkwh7g9 (ref. 41).
References
Eisenlord, M. E. et al. Ochre star mortality during the 2014 wasting disease epizootic: role of population size structure and temperature. Philos. Trans. R. Soc. B 371, 20150212 (2016).
Hewson, I. et al. Densovirus associated with sea-star wasting disease and mass mortality. Proc. Natl Acad. Sci. USA 111, 17278–17283 (2014).
Dawson, M. N. et al. A decade of death and other dynamics: deepening perspectives on the diversity and distribution of sea stars and wasting. Biol. Bull. 244, 143–163 (2023).
Harvell, C. D. et al. Disease epidemic and a marine heat wave are associated with the continental-scale collapse of a pivotal predator (Pycnopodia helianthoides). Sci. Adv. 5, eaau7042 (2019).
Hamilton, S. L. et al. Disease-driven mass mortality event leads to widespread extirpation and variable recovery potential of a marine predator across the eastern Pacific. Proc. R. Soc. B 288, 20211195 (2021).
Gravem, S. A. et al. Sunflower Sea Star (Pycnopodia helianthoides) (IUCN Red List of Threatened Species, 2021).
Lowry, D. et al. Endangered Species Act Status Review Report: Sunflower Sea Star (Pycnopodia helianthoides) (National Marine Fisheries Service, Office of Protected Resources, 2022).
Galloway, A. W. E. et al. Sunflower star predation on urchins can facilitate kelp forest recovery. Proc. R. Soc. B 290, 20221897 (2023).
Heady, W. N. et al. Roadmap to Recovery for the Sunflower Sea Star (Pycnopodia helianthoides) along the West Coast of North America (The Nature Conservancy, 2022).
Hewson, I. et al. Investigating the complex association between viral ecology, environment, and Northeast Pacific sea star wasting. Front. Mar. Sci. 5, 77 (2018).
Hewson, I., Johnson, M. R. & Reyes-Chavez, B. Lessons learned from the sea star wasting disease investigation. Ann. Rev. Mar. Sci. 17, 2.1–2.23 (2025).
Jackson, E. W., Pepe-Ranney, C., Johnson, M. R., Distel, D. L. & Hewson, I. A highly prevalent and pervasive densovirus discovered among sea stars from the North American Atlantic Coast. Appl. Environ. Microbiol. 86, e02723-19 (2020).
Jackson, E. W. et al. Diversity of sea star-associated densoviruses and transcribed endogenous viral elements of densovirus origin. J. Virol. 95, e01594-20 (2021).
Lloyd, M. M. & Pespeni, M. H. Microbiome shifts with onset and progression of sea star wasting disease revealed through time course sampling. Sci. Rep. 8, 16476 (2018).
Aquino, C. A. et al. Evidence that microorganisms at the animal–water interface drive sea star wasting disease. Front. Microbiol. 11, 610009 (2021).
Schiebelhut, L. M., DeBiasse, M. B., Gabriel, L., Hoff, K. J. & Dawson, M. N. A reference genome for ecological restoration of the sunflower sea star, Pycnopodia helianthoides. J. Hered. 115, 86–93 (2023).
Buchfink, B., Reuter, K. & Drost, H.-G. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 18, 366–368 (2021).
Bolyen, E. et al. Reproducible, interactive, scalable, and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857 (2019).
Bokulich, N. A. et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 6, 90 (2018).
McDonald, D. et al. Greengenes2 unifies microbial data in a single reference tree. Nat. Biotechnol. 42, 715–718 (2024).
Lin, H. & Das Peddada, S. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 11, 3514 (2020).
Baker-Austin, C., Trinanes, J., Gonzalez-Escalona, N. & Martinez-Urtaza, J. Non-cholera Vibrios: the microbial barometer of climate change. Trends Microbiol. 25, 76–84 (2017).
Gehman, A.-L. M. et al. Fjord oceanographic dynamics provide refuge for critically endangered Pycnopodia helianthoides. Proc. R. Soc. B 292, 20242770 (2025).
Menge, B. A. et al. Sea star wasting disease in the keystone predator Pisaster ochraceus in Oregon: insights into differential population impacts, recovery, predation rate, and temperature effects from long-term research. PLoS ONE 11, e0153994 (2016).
Suttle, C. A., Chen, F. & Chan, A. M. in International Marine Biotechnology Conference IMBC-91: Short Communications of the Invited Lectures (ed. Nash, C. C.) 153–163 (W. Brown,1992).
Suttle, C. A. & Chen, F. Mechanisms and rates of decay of marine viruses in seawater. Appl. Environ. Microbiol. 58, 3721–3729 (1992).
Middleboe, M., Chan, A. M. & Bertelsen, S. K. in Manual of Aquatic Viral Ecology (eds Wilhelm, S. W. et al.) 118–133 (ASLO, 2010).
Zhong, K. X. et al. Draft genome sequence of Vibrio pectenicida strain FHCF-3, a causative agent of sea star wasting disease in the sunflower sea star (Pycnopodia helianthoides), reveals the genetic potential to produce aerolysin-like toxins. Microbiol. Resour. Announc. (in the press).
Lambert, C., Nicolas, J. L., Cilia, V. & Corre, S. Vibrio pectenicida sp. nov., a pathogen of scallop (Pecten maximus) larvae. Int. J. Syst. Bacteriol. 48, 481–487 (1998).
McCracken, A. R. et al. Microbial dysbiosis precedes signs of sea star wasting disease in wild populations of Pycnopodia helianthoides. Front. Mar. Sci. 10, 1130912 (2023).
Nicolas, J. L., Corre, S., Gauthier, G., Robert, R. & Ansquer, D. Bacterial problems associated with scallop Pecten maximus larval culture. Dis. Aquat. Organ. 27, 67–76 (1996).
Lambert, C. & Nicolas, J. L. Specific inhibition of chemiluminescent activity by pathogenic Vibrios in hemocytes of two marine bivalves: Pecten maximus and Crassostrea gigas. J. Invertebr. Pathol. 71, 53–63 (1998).
Sandlund, N., Torkildsen, L., Magnesen, T., Mortensen, S. & Bergh, Ø. Immunohistochemistry of great scallop Pecten maximus larvae experimentally challenged with pathogenic bacteria. Dis. Aquat. Organ. 69, 163–173 (2006).
Kesarcodi-Watson, A., Miner, P., Nicolas, J. L. & Robert, R. Protective effect of four potential probiotics against pathogen-challenge of the larvae of three bivalves: Pacific oyster (Crassostrea gigas), flat oyster (Ostrea edulis) and scallop (Pecten maximus). Aquaculture 344–349, 29–34 (2012).
Kesarcodi-Watson, A., Miner, P., Nicolas, J. L., Asmani, K. & Robert, R. Pathogenic threats and probiotic use in larviculture of the scallop, Pecten maximus. Aquac. Res. 47, 1221–1230 (2016).
Lambert, C., Nicolas, J. L. & Bultel, V. Toxicity to bivalve hemocytes of pathogenic Vibrio cytoplasmic extract. J. Invertebr. Pathol. 77, 165–172 (2001).
Kehlet-Delgado, H., Häse, C. C. & Mueller, R. S. Comparative genomic analysis of Vibrios yields insights into genes associated with virulence towards C. gigas larvae. BMC Genom. 21, 599 (2020).
GBIF Occurrence Download (GBIF, accessed 5 May 2025); https://doi.org/10.15468/dl.ast4b7
Kanungo, K. in Invertebrate Blood (ed. Cheng, T. C.) 7–39 (Springer, 1984).
Hodin, J., Pearson-Lund, A., Anteau, F. P., Kitaeff, P. & Cefalu, S. Progress toward complete life cycle culturing of the endangered sunflower star, Pycnopodia helianthoides. Biol. Bull. 241, 3 (2021).
Prentice, M. B. et al. Vibrio pectenicida strain FHC F-3 is a causative agent of sea star wasting disease. Dryad https://doi.org/10.5061/dryad.5mkkwh7g9 (2025).
Montecino-Latorre, D. et al. Devastating transboundary impacts of sea star wasting disease on subtidal asteroids. PLoS ONE 11, e0163190 (2016).
Fuess, L. E. et al. Up in arms: immune and nervous system response to sea star wasting disease. PLoS ONE 10, e0133053-16 (2015).
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400 (2017).
Lüdecke, D. sjPlot: Data visualization for statistics in social science. R package version 2.8.17 https://CRAN.R-project.org/package=sjPlot (2024).
Apprill, A., McNally, S., Parsons, R. & Weber, L. Minor revision to V4 region SSU rRNA 806R gene primer greatly increases detection of SAR11 bacterioplankton. Aquat. Microb. Ecol. 75, 129–137 (2015).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data (2010); www.bioinformatics.babraham.ac.uk/projects/fastqc
Ewels, P., Magnusson, M., Lundin, S. & Käller, M. MultiQC: summarize analysis results for multiple tools and samples in a single report. Bioinformatics 32, 3047–3048 (2016).
Li, H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. Preprint at https://arxiv.org/abs/1303.3997 (2013).
Danecek et al. Twelve years of SAMools and BCFtools. Gigascience 10, gia008 (2021).
Bushmanova, E., Antipov, D., Lapidus, A. & Prjibelski, A. D. rnaSPAdes: a de novo transcriptome assembler and its application to RNA-Seq data. Gigascience 8, giz100 (2019).
Huson, D. H. et al. MEGAN Community Edition: interactive exploration and analysis of large-scale microbiome sequencing data. PLoS Comput. Biol. 12, e1004957 (2016).
R Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2024).
Oksanen, J. et al. Vegan: Community ecology package. R package version 2.7-0 https://github.com/vegandevs/vegan (2024).
Wickham, H. ggplot2: Elegant graphics for data analysis. R package version 3.5.1 https://ggplot2.tidyverse.org (2016).
Hester, J. & Bryan, J. glue: Interpreted string literals. R package version 1.8.0 https://glue.tidyverse.org (2024).
Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10 (2011).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Davis, N. M., Proctor, D., Holmes, S. P., Relman, D. A. & Callahan, B. J. Simple statistical identification and removal of contaminant sequences in marker-gene and metagenomics data. Microbiome 6, 226 (2018).
Wick, R. Porechop: adapter trimmer for Oxford Nanopore reads, version 0.2. GitHub https://github.com/rrwick/Porechop (2018).
Wick, R. R., Judd, L. M., Gorrie, C. L. & Holt, K. E. Unicycler: resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 13, e1005595 (2017).
Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. & Tyson, G. W. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055 (2015).
Dong, X. & Strous, M. An integrated pipeline for annotation and visualization of metagenomic contigs. Front. Genet. 10, 999 (2019).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and useability. Mol. Biol. Evol. 30, 772–780 (2013).
Steenwyk, J. L., Buida, T. J. III, Li, Y., Shen, X.-X. & Rokas, A. ClipKIT: a multiple sequence alignment trimming software for accurate phylogenetic inference. PLoS Biol. 18, e3001007 (2020).
Nguyen, L.-T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Yoon, S.-H., Ha, S.-M., Lim, J. M., Kwon, S. J. & Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Anton. Leeuw. 110, 1281–1286 (2017).
Acknowledgements
B. Blake and N. Siu helped to acquire permits for this work. K. Bachen, H. Carson, K. Collins, D. Currie-Olsen, T. Frierson, T. Froese, J. Kocian, E. Loose, Z. Monteith, O. Pontier, G. Sadlier-Brown, A. Schmill, K. Sowul, B. Stevick and D. VanMaanen assisted with field collections. F. Curliss, A. Kalytiak-Davis, C. Schwab and V. Valdez supported sea star transfers from and sample collections at Friday Harbor Laboratories. D. Rogers provided local shellfish to feed experimental stars. J. Beal, C. Grady, J. Gregg, A. MacKenzie and W. Richards provided facilities and logistical support at the USGS Marrowstone Marine Field Station and H. Kuttenkeuler, K. Rolheiser and M. Winningham aided experiment monitoring and sampling. Y. Gouin and A. Nimmon assisted with entering and collating data. C. L. J. Huang assisted with preparation of culture media. C. Burge, C. Conway, J. Hansen, A. Hawthorn, J. Lovy, A. Roberts and Q. Yang provided advice. Graphic illustrations are credited to M. Minck. We are grateful for the support from E. Peterson, C. Munck, N. Eddy and J. Wilson. Funding was provided by The Nature Conservancy of California (C.D.H. and A.-L.M.G.), the Tula Foundation (A.-L.M.G. and C.A.S.), the Natural Sciences and Engineering Research Council of Canada Discovery grant no. RPGIN-2020-06515 (C.A.S.), the Canadian Foundation for Innovation and British Columbia Knowledge Development Fund Infrastructure award no. 25412 (C.A.S.), the University of British Columbia, the USGS Biological Threats Research Program, Ecosystems Mission Area (P.K.H.) and the Quantitative and Evolutionary STEM Traineeship NRT-1735316 (A.M.). Any use of trade, firm or product names is for descriptive purposes only and does not imply endorsement by the US government.
Author information
Authors and Affiliations
Contributions
Conceptualization: M.B.P., G.A.C., A.M.C., K.M.D., P.K.H., J.F.F., C.D.H., C.A.S., A.-L.M.G. Methodology: M.B.P., G.A.C., A.M.C., K.M.D., P.K.H., C.T.E.K., C.D.H., C.A.S., A.-L.M.G. Formal analysis: M.B.P., A.M.C., K.X.Z., A.-L.M.G. Investigation: M.B.P., G.A.C., A.M.C., K.M.D., A.M., R.B.G.C.-C., C.P., A.-L.M.G. Resources: M.B.P., G.A.C., K.M.D., P.K.H., J.H., A.M., C.A.S., A.-L.M.G. Data curation: M.B.P., G.A.C., A.M.C., K.M.D., C.P., A.-L.M.G. Writing—original draft: M.B.P., A.-L.M.G. Writing—review and editing: M.B.P., G.A.C., A.M.C., K.M.D., P.K.H., J.F.F., J.H., A.M., C.T.E.K., R.B.G.C.-C., C.P., K.X.Z., C.D.H., C.A.S., A.-L.M.G. Visualization: M.B.P., K.X.Z., A.-L.M.G. Supervision: A.M.C., C.D.H., C.A.S., A.-L.M.G. Funding acquisition: A.M.C., C.D.H., C.A.S., A.-L.M.G.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Ecology & Evolution thanks Kevin Lafferty and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 The disease trajectory of captive-bred (“lab”, n = 4) and wild (“wild”, n = 4) sunflower sea stars (Pycnopodia helianthoides) following injection with coelomic fluid from a diseased sunflower sea star.
Data represents 8 individual mortalities observed in sunflower sea stars exposed to sea star wasting disease (excluding 2 individuals that were exposed but did not die). Host responses are grouped into three types, arm twisting (‘twist’), arm autotomy (‘drop’) and mortality (‘mort’). Symbols indicate the average number of days post exposure when disease responses were observed. The error bars indicate 1 s.e. We confirmed the presence of V. pectenicida in the inoculum used to start this experiment using 16S rRNA gene amplicon sequencing (Supplementary Table 11).
Extended Data Fig. 2 Model predicted values of the number of arms twisted in sunflower sea stars (Pycnopodia helianthoides) following controlled exposure to sea star wasting disease by exposure method.
Lines indicate adjusted predicted values for stars within exposed (blue) and control (orange) treatment groups with 95% confidence intervals. Exposure methods include stars injected with coelomic fluid (‘CF’), tissue homogenate (‘homogenate’), or Vibrio pectenicida culture (‘culture’), and stars exposed to effluent water from a wasting sea stars tank (‘water’). Statistical results can be found in Supplementary Table 3.
Extended Data Fig. 3 Phylogenetic relationship of 16S rRNA gene sequence of V. pectenicida strain FHCF-3 (this study) to other Vibrio spp.
Vibrio spp. 16S rRNA gene sequences were retrieved from the SILVA rRNA gene database (version 138.1) and the NCBI nr database (as of 14 November 2024). The maximum-likelihood phylogeny was built using 1,000 replicates, and rooted with sequences from two strains of Escherichia coli (NCBI Accessions CP033092 and MT215717) as an outgroup. Values on the nodes of the phylogeny represent bootstrap values. V. pectenicida strain FHCF-3 (isolated and sequenced in this study) is highlighted in red.
Supplementary information
Supplementary Information
Supplementary Tables 1–11.
Supplementary Data 1
Supplementary data.
Supplementary Data 2
Supplementary data.
Supplementary Data 3
Supplementary data.
Source data
Source Data Fig. 2
Source data.
Source Data Fig. 3
Source data.
Source Data Fig. 4
Source data.
Source Data Extended Data Fig. 1
Source data.
Source Data Extended Data Fig. 2
Source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Prentice, M.B., Crandall, G.A., Chan, A.M. et al. Vibrio pectenicida strain FHCF-3 is a causative agent of sea star wasting disease. Nat Ecol Evol 9, 1739–1751 (2025). https://doi.org/10.1038/s41559-025-02797-2
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41559-025-02797-2
This article is cited by
-
Mystery of billions of sea-star deaths solved at last
Nature (2025)
-
Sea star wasting disease mystery finally solved
Nature Ecology & Evolution (2025)