Abstract
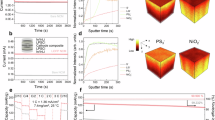
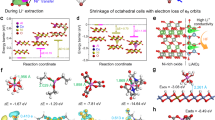
All-solid-state batteries (ASSBs) comprising Ni-rich layered cathode active materials (CAMs) and sulfide solid electrolytes are promising candidates for next-generation batteries with high energy densities and safety. However, severe capacity fading occurs due to surface degradation at the CAM–electrolyte interface and severe lattice volume changes in the CAM, resulting in inner-particle isolation and detachment of the CAM from the electrolyte. Here we quantified the capacity fading factors of Ni-rich Li[NixCoyAl1−x−y]O2 composite ASSB cathodes as functions of Ni content. Surface degradation at the CAM–electrolyte interface was found to be the main cause of capacity fading in a CAM with 80% Ni content, whereas inner-particle isolation and detachment of the CAM from the electrolyte play a substantial role as the Ni content increases to 85% or more. On the basis of the comprehensive understanding of these mechanisms in ASSBs, high-performance Ni-rich CAMs with columnar structures were developed through surface and morphology modification.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its Supplementary Information. Source data are provided with this paper.
References
Noh, H.-J., Youn, S., Yoon, C. S. & Sun, Y.-K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8 and 0.85) cathode material for lithium-ion batteries. J. Power Sources 233, 121–130 (2013).
de Biasi, L. et al. Between scylla and charybdis: balancing among structural stability and energy density of layered NCM cathode materials for advanced lithium-ion batteries. J. Phys. Chem. C 121, 26163–26171 (2017).
Watanabe, S., Kinoshita, M., Hosokawa, T., Morigaki, K. & Nakura, K. Capacity fade of LiAlyNi1-x-yCoxO2 cathode for lithium-ion batteries during accelerated calendar and cycle life tests (surface analysis of LiAlyNi1-x-yCoxO2 cathode after cycle tests in restricted depth of discharge ranges). J. Power Sources 258, 210–217 (2014).
Miller, D. J., Proff, C., Wen, J. G., Abraham, D. P. & Bareño Observation of microstructural evolution in Li battery cathode oxide particles by in situ electron microscopy. Adv. Energy Mater. 3, 1098–1103 (2013).
Kondrakov, A. O. et al. Anisotropic lattice strain and mechanical degradation of high- and low-nickel NCM cathode materials for Li-ion batteries. J. Phys. Chem. C 121, 3286–3294 (2017).
Ryu, H.-H., Park, K.-J., Yoon, C. S. & Sun, Y.-K. Capacity fading of Ni-rich Li[NixCoyMn1-x-y]O2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: bulk or surface degradation? Chem. Mater. 30, 1155–1163 (2018).
Park, N.-Y. et al. Degradation mechanism of Ni-rich cathode materials: focusing on particle interior. ACS Energy Lett. 7, 2362–2369 (2022).
Bak, S.-M. et al. Structural changes and thermal stability of charged LiNixMnyCozO2 cathode materials studied by combined in situ time-resolved XRD and mass spectroscopy. ACS Appl. Mater. Interfaces 6, 22594–22601 (2014).
Sakuda, A., Hayashi, A. & Tatsumisago, M. Sulfide solid electrolyte with favorable mechanical property for all-solid-state lithium battery. Sci. Rep. 3, 2261 (2013).
Zhu, Y., He, X. & Mo, Y. Origin of outstanding stability in the lithium solid electrolyte materials: insights from thermodynamic analyses based on first-principles calculations. ACS Appl. Mater. Interfaces 7, 23685–23693 (2015).
Schweidler, S. et al. Investigation into mechanical degradation and fatigue of high-Ni NCM cathode material: a long-term cycling study of full cells. ACS APpl. Energy Mater. 2, 7375–7384 (2019).
Yu, T.-Y. et al. Limitation of Ni-rich layered cathodes in all-solid-state lithium batteries. J. Mater. Chem. A 11, 24629–24636 (2023).
Kim, A.-Y. et al. Effect of surface carbonates on the cyclability of LiNbO3-coated NCM622 in all-solid-state batteries with lithium thiophosphate electrolytes. Sci. Rep. 11, 5367 (2021).
Culver, S. P., Koerver, R., Zeier, W. G. & Janek, J. On the functionality of coatings for cathode active materials in thiophosphate-based all-solid-state batteries. Adv. Energy Mater. 9, 1900626 (2019).
Kitsche, D. et al. Atomic layer deposition derived zirconia coatings on Ni-rich cathodes in solid-state batteries: correlation between surface constitution and cycling performance. Small Sci. 3, 2200073 (2023).
Ruess, R. et al. Influence of NCM particle cracking on kinetics of lithium-ion batteries with liquid or solid electrolyte. J. Electrochem. Soc. 167, 100532 (2020).
Teo, J. H. et al. The interplay between (electro)chemical and (chemo)mechanical effects in the cycling performance of thiophosphate-based solid-state batteries. Mater. Futures 1, 015102 (2022).
Koerver, R. et al. Capacity fade in solid-state batteries: interphase formation and chemomechanical processes in nickel-rich layered oxide cathodes and lithium thiophosphate solid electrolytes. Chem. Mater. 29, 5574–5582 (2017).
Wu, E. A. et al. Facile, dry-processed lithium borate-based cathode coating for improved all-solid-state battery performance. J. Electrochem. Soc. 167, 130516 (2020).
Kim, U.-H. et al. Microstructure- and interface-modified Ni-rich cathode for high-energy-density all-solid-state lithium batteries. ACS Energy Lett. 8, 809–817 (2023).
Haruyama, J., Sodeyama, K., Han, L., Takada, T. & Tateyama, Y. Space-charge layer effect at interface between oxide cathode and sulfide electrolyte in all-solid-state lithium-ion battery. Chem. Mater. 26, 4248–4255 (2014).
Wang, L. et al. In-situ visualization of the space-charge-layer effect on interfacial lithium-ion transport in all-solid-state batteries. Nat. Commun. 11, 5889 (2020).
Park, N.-Y. et al. Mechanism of doping with high‐valence elements for developing Ni‐rich cathode materials. Adv. Energy Mater. 13, 2301530 (2023).
Nunes, B. N. et al. The role of niobium in layered oxide cathodes for conventional lithium-ion and solid-state batteries. Inorg. Chem. Front. 10, 7126–7145 (2023).
Ryu, H.-H. et al. A highly stabilized Ni-rich NCA cathode for high-energy lithium-ion batteries. Mater. Today 36, 73–82 (2020).
Jung, S. H. et al. Ni‐rich layered cathode materials with electrochemo‐mechanically compliant microstructures for all‐solid‐state Li batteries. Adv. Energy Mater. 10, 1903360 (2020).
Nam, G. W. et al. Capacity fading of Ni-rich NCA cathodes: effect of microcracking extent. ACS Energy Lett. 4, 2995–3001 (2019).
Kim, A.-Y. et al. Stabilizing effect of a hybrid surface coating on Ni-rich NCM cathode material in all-solid-state batteries. Chem. Mater. 31, 9664–9672 (2019).
Strauss, F. et al. Li2ZrO3-coated NCM622 for application in inorganic solid-state batteries: role of surface carbonates in the cycling performance. ACS Appl. Mater. Interfaces 12, 57146–57154 (2020).
Walther, F. et al. The working principle of a Li2CO3/LiNbO3 coating on NCM for thiophosphate-based all-solid-state batteries. Chem. Mater. 33, 2110–2125 (2021).
Ma, Y. et al. Cycling performance and limitations of LiNiO2 in solid-state batteries. ACS Energy Lett. 6, 3020–3028 (2021).
Koerver, R. et al. Redox-active cathode interphases in solid-state batteries. J. Mater. Chem. A 5, 22750–22760 (2017).
Sumita, M., Tanaka, Y. & Ohno, T. Possible polymerization of PS4 at a Li3PS4/FePO4 interface with reduction of the FePO4 phase. J. Phys. Chem. C 121, 9698–9704 (2017).
Richards, W. D., Miara, L. J., Wang, Y., Kim, J. C. & Ceder, G. Interface stability in solid-state batteries. Chem. Mater. 28, 266–273 (2016).
Zhou, L. et al. High areal capacity, long cycle life 4 V ceramic all-solid-state Li-ion batteries enabled by chloride solid electrolytes. Nat. Energy 7, 83–93 (2022).
Chun, G. H., Shim, J. H. & Yu, S. Computational investigation of the interfacial stability of lithium chloride solid electrolytes in all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 14, 1241–1248 (2022).
Rosenbach, C. et al. Visualizing the chemical incompatibility of halide and sulfide-based electrolytes in solid-state batteries. Adv. Energy Mater. 13, 2203673 (2023).
Kochetkov, I. et al. Different interfacial reactivity of lithium metal chloride electrolytes with high voltage cathodes determines solid-state battery performance. Energy Environ. Sci. 15, 3933–3944 (2022).
Gao, X. et al. Solid-state lithium battery cathodes operating at low pressures. Joule 6, 636–646 (2022).
Zeier, W. G. & Janek, J. Challenges in speeding up solid-state battery development. Nat. Energy 8, 230–240 (2023).
Koerver, R. et al. Chemo-mechanical expansion of lithium electrode materials—on the route to mechanically optimized all-solid-state batteries. Energy Environ. Sci. 11, 2142–2158 (2018).
Strauss, F. et al. Operando characterization techniques for all-solid-state lithium-ion batteries. Adv. Energy Sustainability Res. 2, 2100004 (2021).
Jung, Y.-C. et al. On-site formation of silver decorated carbon as an anodeless electrode for high-energy density all-solid-state batteries. J. Mater. Chem. A 11, 25275–25282 (2023).
Strauss, F. et al. Rational design of quasi-zero-strain NCM cathode materials for minimizing volume change effects in all-solid-state batteries. ACS Mater. Lett. 2, 84–88 (2020).
Mücke, R. et al. Modelling electro-chemically induced stresses in all-solid-state batteries: screening electrolyte and cathode materials in composite cathodes. J. Mater. Chem. A 11, 18801–18810 (2023).
Bucci, G., Swamy, T., Chiang, Y.-M. & Carter, W. C. Modeling of internal mechanical failure of all-solid-state batteries during electrochemical cycling, and implications for battery design. J. Mater. Chem. A 5, 19422–19430 (2017).
Acknowledgements
We thank W. Cho (Advanced Batteries Research Center, Korea Electronics Technology Institute) for pouch-type full-cell tests with a dry-processed electrode. This work was supported by the Human Resources Development Program (number 20214000000320; N.-Y.P., S.-M.P., Y.-K.S.) and ESS Big Data-Based O&M and Asset Management Technical Manpower Training (RS-2024-00398346; N.-Y.P., I.-S.L., Y.-K.S.) of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), funded by the Ministry of Trade, Industry and Energy of the Korean government.
Author information
Authors and Affiliations
Contributions
Y.-K.S. conceived and designed the research. H.-U.L., I.-S.L., S.-M.P., Y.-C.J. performed the experiments and characterization of materials. N.-Y.P., H.-U.L., T.-Y.Y, H.K. and H.-G.J. analysed the data. N.-Y.P., H.-U.L., T.-Y.Y, H.K. and Y.-K.S. contributed to the discussion of the results. N.-Y.P., T.-Y.Y. and H.K. wrote the original draft. N.-Y.P. and Y.-K.S. reviewed and edited the paper. All the authors commented on and revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–20, Tables 1–3, References 1–18.
Supplementary Data 1
Source data for Supplementary Information.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, NY., Lee, HU., Yu, TY. et al. High-energy, long-life Ni-rich cathode materials with columnar structures for all-solid-state batteries. Nat Energy 10, 479–489 (2025). https://doi.org/10.1038/s41560-025-01726-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41560-025-01726-8
This article is cited by
-
Research on battery technology at Hanyang University
Nature Reviews Electrical Engineering (2026)
-
Exploring Degradation Mechanisms and Recent Developments in High-Nickel Layered Cathodes for Lithium Batteries
Electrochemical Energy Reviews (2025)
-
Insights into effects of Co and Mn elements on the cycle stability of ultrahigh-nickel cathodes charged at same delithiation state
Rare Metals (2025)