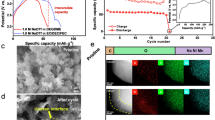
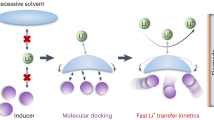
Abstract
Fast charging of high-energy batteries is critical for transportation electrification but remains challenging because the rapid rise in cell overpotential easily exceeds electrolytes’ fixed electrochemical stability window. Here we design a self-adaptive electrolyte with a dynamically expanding electrochemical stability window that increases in real time during charging, outpacing the rise in overpotential as the charging current intensifies. The self-adaptive electrolyte is a single-phase solution of salt and complementary oxidation- and reduction-resistant solvents at the cloud point composition but can undergo solvent separation to dynamically redistribute solvent components during charging. The oxidation-resistant solvents concentrate at the positive electrode and reduction-resistant solvents accumulate at the negative electrode, broadening the electrolyte stability window in real time during charging. Proof-of-concept experiments validate the versatility of this design in both aqueous zinc-metal and non-aqueous lithium-metal batteries, achieving high Coulombic efficiencies of negative electrodes and enhanced oxidative stability for positive electrodes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this article and its Supplementary Information. Source data are provided with this paper.
References
Xu, K. Electrolytes and interphases in Li-ion batteries and beyond. Chem. Rev. 114, 11503–11618 (2014).
Moshkovich, M., Gofer, Y. & Aurbach, D. Investigation of the electrochemical windows of aprotic alkali metal (Li, Na, K) salt solutions. J. Electrochem. Soc. 148, E155 (2001).
Jin, Y. et al. Low-solvation electrolytes for high-voltage sodium-ion batteries. Nat. Energy 7, 718–725 (2022).
Zheng, W. et al. Enhancing the reaction kinetics and structural stability of high-voltage LiCoO2 via polyanionic species anchoring. Energy Environ. Sci. 17, 4147–4156 (2024).
Bi, Y. et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 370, 1313–1317 (2020).
Suo, L. et al. ‘Water-in-salt’ electrolyte enables high-voltage aqueous lithium-ion chemistries. Science 350, 938–943 (2015).
Kühnel, R.-S., Reber, D. & Battaglia, C. Perspective—electrochemical stability of water-in-salt electrolytes. J. Electrochem. Soc. 167, 070544 (2020).
Xu, K. Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chem. Rev. 104, 4303–4418 (2004).
Liang, X. et al. A facile surface chemistry route to a stabilized lithium metal anode. Nat. Energy 2, 17119 (2017).
Wan, H., Xu, J. & Wang, C. Designing electrolytes and interphases for high-energy lithium batteries. Nat. Rev. Chem. 8, 30–44 (2024).
Lv, T. & Suo, L. Water-in-salt widens the electrochemical stability window: thermodynamic and kinetic factors. Curr. Opin. Electrochem. 29, 100818 (2021).
Ye, Y. et al. Quadruple the rate capability of high-energy batteries through a porous current collector design. Nat. Energy 9, 643–653 (2024).
Landesfeind, J., Hattendorff, J., Ehrl, A., Wall, W. A. & Gasteiger, H. A. Tortuosity determination of battery electrodes and separators by impedance spectroscopy. J. Electrochem. Soc. 163, A1373 (2016).
Lu, L.-L. et al. Extremely fast-charging lithium ion battery enabled by dual-gradient structure design. Sci. Adv. 8, eabm6624 (2022).
Parikh, D., Christensen, T. & Li, J. Correlating the influence of porosity, tortuosity, and mass loading on the energy density of LiNi0.6Mn0.2Co0.2O2 cathodes under extreme fast charging (XFC) conditions. J. Power Sources 474, 228601 (2020).
Lu, Y. et al. Breaking the molecular symmetricity of sulfonimide anions for high-performance lithium-metal batteries under extreme cycling conditions. Nat. Energy https://doi.org/10.1038/s41560-024-01679-4 (2024).
Li, T., Zhang, X.-Q., Shi, P. & Zhang, Q. Fluorinated solid-electrolyte interphase in high-voltage lithium-metal batteries. Joule 3, 2647–2661 (2019).
Zhang, Q.-K. et al. Homogeneous and mechanically stable solid–electrolyte interphase enabled by trioxane-modulated electrolytes for lithium-metal batteries. Nat. Energy 8, 725–735 (2023).
Jain, R. et al. Nanostructuring versus microstructuring in battery electrodes. Nat. Rev. Mater. 7, 736–746 (2022).
Kim, Y. et al. Sodium biphenyl as anolyte for sodium–seawater batteries. Adv. Funct. Mater. 30, 2001249 (2020).
Cho, S.-J. et al. Monolithic heterojunction quasi-solid-state battery electrolytes based on thermodynamically immiscible dual phases. Energy Environ. Sci. 12, 559–565 (2019).
Park, N. R. et al. Understanding the role of lithium borate as the surface coating on high-voltage single crystal LiNi0.5Mn1.5O4. Adv. Funct. Mater. 34, 2312091 (2024).
Jafari, S. A. & Entezari, M. H. Salting out in ACN/water systems: Hofmeister effects and partition of quercetin. J. Mol. Liq. 312, 113331 (2020).
Li, M., Zhuang, B., Lu, Y., An, L. & Wang, Z.-G. Salt-induced liquid–liquid phase separation: combined experimental and theoretical investigation of water–acetonitrile–salt mixtures. J. Am. Chem. Soc. 143, 773–784 (2021).
Hyde, A. M. et al. General principles and strategies for salting-out informed by the Hofmeister series. Org. Process Res. Dev. 21, 1355–1370 (2017).
Jiao, Y. et al. Mechanical bond-assisted full-spectrum investigation of radical interactions. J. Am. Chem. Soc. 144, 23168–23178 (2022).
Thomas, A. P., Palanikumar, L., Jeena, M., Kim, K. & Ryu, J.-H. Cancer-mitochondria-targeted photodynamic therapy with supramolecular assembly of HA and a water soluble NIR cyanine dye. Chem. Sci. 8, 8351–8356 (2017).
Gomes, R. J. et al. Modulating water hydrogen bonding within a non-aqueous environment controls its reactivity in electrochemical transformations. Nat. Catal. 7, 689–701 (2024).
Ohno, K., Okimura, M., Akai, N. & Katsumoto, Y. The effect of cooperative hydrogen bonding on the OH stretching-band shift for water clusters studied by matrix-isolation infrared spectroscopy and density functional theory. Phys. Chem. Chem. Phys. 7, 3005–3014 (2005).
Jie, Y. et al. Enabling high-voltage lithium-metal batteries by manipulating solvation structure in ester electrolyte. Angew. Chem. Int. Ed. 59, 3505–3510 (2020).
Aurbach, D., Youngman, O., Gofer, Y. & Meitav, A. The electrochemical behaviour of 1,3-dioxolane—LiClO4 solutions—I. uncontaminated solutions. Electrochim. Acta 35, 625–638 (1990).
Adams, B. D., Zheng, J., Ren, X., Xu, W. & Zhang, J.-G. Accurate determination of Coulombic efficiency for lithium metal anodes and lithium-metal batteries. Adv. Energy Mater. 8, 1702097 (2018).
Becke, A. D. Density‐functional thermochemistry. I. the effect of the exchange‐only gradient correction. J. Chem. Phys. 96, 2155–2160 (1992).
Frisch, M. J. et al. Gaussian 09, revision A.02 (Gaussian Inc., 2009).
Plimpton, S. Fast parallel algorithms for short-range molecular-dynamics. J. Comput. Phys. 117, 1–19 (1995).
Jorgensen, W. L., Maxwell, D. S. & TiradoRives, J. Development and testing of the OPLS all-atom force field on conformational energetics and properties of organic liquids. J. Am. Chem. Soc. 118, 11225–11236 (1996).
Kaminski, G. A., Friesner, R. A., Tirado-Rives, J. & Jorgensen, W. L. Evaluation and reparametrization of the OPLS-AA force field for proteins via comparison with accurate quantum chemical calculations on peptides. J. Phys. Chem. B 105, 6474–6487 (2001).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Dodda, L. S., de Vaca, I. C., Tirado-Rives, J. & Jorgensen, W. L. LigParGen web server: an automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 45, W331–W336 (2017).
Marenich, A. V., Jerome, S. V., Cramer, C. J. & Truhlar, D. G. Charge model 5: an extension of hirshfeld population analysis for the accurate description of molecular interactions in gaseous and condensed phases. J. Chem. Theory Comput. 8, 527–541 (2012).
Vilseck, J. Z., Tirado-Rives, J. & Jorgensen, W. L. Evaluation of CM5 charges for condensed-phase modeling. J. Chem. Theory Comput. 10, 2802–2812 (2014).
Su, Q. et al. Organic fillers regulating the solvation structure of zinc ions in aqueous electrolytes for high-stability Zn2+/Li+ hybrid batteries. ACS Appl. Energy Mater. 6, 11299–11307 (2023).
Zeron, I. M., Abascal, J. L. F. & Vega, C. A force field of Li+, Na+, K+, Mg2+, Ca2+, Cl−, and SO42− in aqueous solution based on the TIP4P/2005 water model and scaled charges for the ions. J. Chem. Phys. 151, 134504 (2019).
Martinez, L., Andrade, R., Birgin, E. G. & Martinez, J. M. PACKMOL: a package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 30, 2157–2164 (2009).
Momma, K. & Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 44, 1272–1276 (2011).
Acknowledgements
This work was supported by the US Department of Energy, Basic Energy Science (award number DE-SC0023408).
Author information
Authors and Affiliations
Contributions
C.-X.Z. and C.W. conceived the idea for the project. C.-X.Z. and Z.L. performed electrochemical experiments. C.-X.Z. conducted the calculation. B.C. and F.C. carried out the NMR analysis. C.-X.Z. and C.W. drafted the paper. C.W. directed the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Elie Paillard and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–29.
Source data
Source Data Fig. 2
Raw data of ternary phase diagrams.
Source Data Fig. 3
Raw data of ternary phase diagrams, ACN content and IR results.
Source Data Fig. 4
Raw data of HER kinetics.
Source Data Fig. 6
Raw data of ternary phase diagrams.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, CX., Li, Z., Chen, B. et al. Self-adaptive electrolytes for fast-charging batteries. Nat Energy 10, 904–913 (2025). https://doi.org/10.1038/s41560-025-01801-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41560-025-01801-0