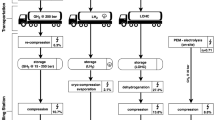
Abstract
Industrially, hydrogen production often relies on carbon-based resources, necessitating the separation of hydrogen from impurities such as CO, CO2, hydrocarbons and N2. Traditional purification methods involve complicated and energy-intensive sequential conversion and removal of these impurities. Here we introduce a reversible catalytic cycle based on the interconversion between γ-butyrolactone and 1,4-butanediol over an inverse Al2O3/Cu catalyst, enabling efficient hydrogen separation and storage from crude hydrogen feeds. This process could transform crude hydrogen feeds containing over 50% impurities into pure hydrogen at low temperature. The low impurity affinity and high dispersion of inverse Al2O3/Cu facilitate catalytic crude and waste hydrogen separations previously considered unachievable. This approach avoids the need for expensive pressure swing adsorption or membrane systems in liquid organic hydrogen carriers, showing great potential for large-scale applications in crude hydrogen or industrial tail gas utilization processes. By providing a low-risk, energy-efficient alternative, this strategy supports the global transition from grey/blue hydrogen to green hydrogen.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper and Supplementary Information files. Source data are provided with this paper.
References
The Future of Hydrogen (International Energy Agency, 2019).
Medium and Long-Term Plan for the Development of Hydrogen Energy Industry (2021–2035) (National Energy Administration, National Development and Reform Commission, 2022).
Lopez, G. et al. Hydrogen generation from biomass by pyrolysis. Nat. Rev. Methods Prim. 2, 20–32 (2022).
Kura, C. et al. Hydrogen separation by nanocrystalline titanium nitride membranes with high hydride ion conductivity. Nat. Energy 2, 786–794 (2017).
Li, S., Lin, L., Wang, Z. & Ma, D. Direct utilization of crude and waste H2 via CO-tolerant hydrogenation. Innovation 4, 100353–100354 (2023).
Eberle, U., Felderhoff, M. & Schuth, F. Chemical and physical solutions for hydrogen storage. Angew. Chem. Int. Ed. 48, 6608–6630 (2009).
Föhlisch, A. et al. The bonding of CO to metal surfaces. J. Chem. Phys. 112, 1946–1958 (2000).
Cao, L. et al. Atomically dispersed iron hydroxide anchored on Pt for preferential oxidation of CO in H2. Nature 565, 631–635 (2019).
Fu, Q. et al. Interface-confined ferrous centers for catalytic oxidation. Science 328, 1141–1144 (2010).
Lin, L. et al. A highly CO-tolerant atomically dispersed Pt catalyst for chemoselective hydrogenation. Nat. Nanotechnol. 14, 354–361 (2019).
Wang, Z. et al. CO-tolerant RuNi/TiO2 catalyst for the storage and purification of crude hydrogen. Nat. Commun. 13, 4404 (2022).
Jorschick, H., Vogl, M., Preuster, P., Bosmann, A. & Wasserscheid, P. Hydrogenation of liquid organic hydrogen carrier systems using multicomponent gas mixtures. Int. J. Hydrog. Energy 44, 31172–31182 (2019).
Qasmi, C. et al. GBL/BDO pair as a bio-based liquid organic carrier: kinetic modeling of liquid phase hydrogenation and dehydrogenation over copper-based catalyst. Int. J. Hydrog. Energy 67, 829–841 (2024).
Chen, J. Technology and Engineering of Adsorption Separation (Science Press, 2022).
Xu, J., Yang, Y. & Li, Y.-W. Recent development in converting coal to clean fuels in China. Fuel 152, 122–130 (2015).
Kattel, S., Ramírez, P. J., Chen, J. G., Rodriguez, J. A. & Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 355, 1296–1299 (2017).
Behrens, M. et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 336, 893–897 (2012).
Kong, X., Chen, Y., Bao, X. & Zhu, Y. Integrating operando spectroscopies and transient analysis for dynamic catalytic insights. Sci. China Chem. https://doi.org/10.1007/s11426-024-2455-1 (2025).
Eblagon, K. M. & Tsang, S. C. E. Structure-reactivity relationship in catalytic hydrogenation of heterocyclic compounds over ruthenium black-part A: effect of substitution of pyrrole ring and side chain in N-heterocycles. Appl. Catal. B 160, 22–34 (2014).
Cui, X. et al. Highly selective hydrogenation of arenes using nanostructured ruthenium catalysts modified with a carbon-nitrogen matrix. Nat. Commun. 7, 11326 (2016).
Eblagon, K. M., Tam, K. & Tsang, S. C. E. Comparison of catalytic performance of supported ruthenium and rhodium for hydrogenation of 9-ethylcarbazole for hydrogen storage applications. Energy Environ. Sci. 5, 8621–8630 (2012).
Yu, H. et al. LaNi5.5 particles for reversible hydrogen storage in N-ethylcarbazole. Nano Energy 80, 105476–105483 (2021).
Zhu, T. et al. A highly active bifunctional Ru-Pd catalyst for hydrogenation and dehydrogenation of liquid organic hydrogen carriers. J. Catal. 378, 382–391 (2019).
Peters, W. et al. Efficient hydrogen release from perhydro-N-ethylcarbazole using catalyst-coated metallic structures produced by selective electron beam melting. Energy Environ. Sci. 8, 641–649 (2015).
Chen, L. et al. Reversible dehydrogenation and rehydrogenation of cyclohexane and methylcyclohexane by single-site platinum catalyst. Nat. Commun. 13, 1092 (2022).
Kustov, L. M., Tarasov, A. L. & Tarasov, B. P. Intermetallide catalysts for hydrogen storage on the basis of reversible aromatics hydrogenation/dehydrogenation reactions. Int. J. Hydrog. Energy 38, 5713–5716 (2013).
Chauhan, A., Kar, A. K. & Srivastava, R. Ru-decorated N-doped carbon nanoflakes for selective hydrogenation of levulinic acid to γ-valerolactone and quinoline to tetrahydroquinoline with HCOOH in water. Appl. Catal. A 636, 118580–118590 (2022).
Sridharan, V., Suryavanshi, P. A. & Menendez, J. C. Advances in the chemistry of tetrahydroquinolines. Chem. Rev. 111, 7157–7259 (2011).
Jee, J. G., Kim, M. B. & Lee, C. H. Adsorption characteristics of hydrogen mixtures in a layered bed: binary, ternary, and five-component mixtures. Ind. Eng. Chem. Res. 40, 868–878 (2001).
Song, C., Liu, Q., Ji, N., Kansha, Y. & Tsutsumi, A. Optimization of steam methane reforming coupled with pressure swing adsorption hydrogen production process by heat integration. Appl. Energy 154, 392–401 (2015).
Liu, J., Jiang, Y., Zhang, X., Fu, L. & Deng, M. Performance optimization of an HT-PEMFC and PSA integrated system with impure hydrogen containing CO2. Appl. Therm. Eng. 214, 118859–118870 (2022).
Yuan, D. et al. Confined Mn2+ enables effective aerobic oxidation catalysis. Sci. China Chem. 67, 1545–1553 (2024).
Yang, C. et al. Homolytic H2 dissociation for enhanced hydrogenation catalysis on oxides. Nat. Commun. 15, 540 (2024).
Huang, Z. W. et al. Hydrogenation of γ-butyrolactone to 1,4-butanediol over CuCo/TiO2 bimetallic catalysts. ACS Catal. 7, 8429–8440 (2017).
Acknowledgements
This work was supported by the Ministry of Science and Technology of China (2023YFA1509103, 2022YFA1503804, Yifeng Zhu; 2021YFA1501102, D.M.; 2023YFA1506602, M.P.), National Natural Science Foundation of China (22272031, 22472035, 22102033, Yifeng Zhu; 22232001, D.M.; 22402035, C.Y.), Science and Technology Commission of Shanghai Municipality (22ZR1408000, 22QA1401300, Yifeng Zhu) and the Fundamental Research Funds for the Central Universities (20720220008, Yifeng Zhu). D.M. acknowledges support from the Tencent Foundation through the XPLORER PRIZE. We are grateful for the support of BL02B01 (31124.02.SSRF.BL02B01), BL05U (31124.02.SSRF.BL05U) and BL06B (31124.02.SSRF.BL06B) at the SSRF. We acknowledge the Energy Strategy and Engineering Consulting Center at the Institute of Coal Chemistry for their support in the techno-economic assessments and X. Duan and Y. Cao from the School of Chemical Engineering, East China University of Science and Technology, for their assistance with Aspen ONE.
Author information
Authors and Affiliations
Contributions
Yifeng Zhu, D.M. and X.B. conceived of the idea and concept. Y. Chen, X.K., C.Y. and G.G. performed the experiments. Y. Chen, X.P., R.M., Y.L. and Yifeng Zhu performed techno-economic and life-cycle assessments. W.S., F.Y., H. Zhang and Z.L. helped with the surface characterizations. Data were discussed among all co-authors. The original draft was organized by Yifeng Zhu, X.K. and Y. Cao. The paper was reviewed and edited by H. Zheng, Yulei Zhu, H. Zhang, M.P., D.M. and X.B., with contributions from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Frederic Meunier and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary materials and methods, Figs. 1–27 and Tables 1–26.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Y., Kong, X., Yang, C. et al. A catalytic cycle that enables crude hydrogen separation, storage and transportation. Nat Energy 10, 971–980 (2025). https://doi.org/10.1038/s41560-025-01806-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41560-025-01806-9
This article is cited by
-
Fick Diffusivity, Thermal Diffusivity, Thermal Conductivity, Density, and Mixture Composition of Binary Mixtures of γ-Butyrolactone and 1,4-Butanediol by Light Scattering Techniques and Conventional Methods
International Journal of Thermophysics (2026)
-
In situ coating strategy for flexible all-perovskite tandem modules
Nature Photonics (2025)
-
Stabilizing dual-phased perovskite towards high performance photovoltaics with enhanced batch stability and consistency
Nature Communications (2025)
-
Impurity-tolerant catalysis
Nature Energy (2025)
-
Microstructural disorder in perovskite photovoltaics
Frontiers of Chemical Science and Engineering (2025)