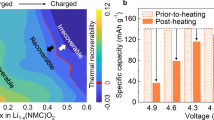
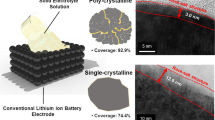
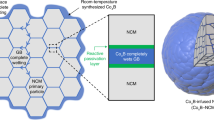
Abstract
Single crystallization remains a debated strategy for advancing Ni-rich cathode materials. While it mitigates particle cracking and improves tap density by eliminating particle boundaries, extended diffusion pathways introduce volumetric and lattice distortions, compromising electrochemical and structural stability. These challenges hinder the commercialization of high-Ni single-crystal cathodes, calling for a reassessment of their viability. Here we report a structural design: intralattice-bonded phase single-crystal LiNi0.92Co0.03Mn0.05O2 (IBP-SC92). This architecture maintains structural integrity while shortening diffusion pathways, resulting in almost zero electrochemical degradation during cycling. The robust structure and fast ion transport mitigate lattice strain, as confirmed by multiscale high-resolution diffraction and imaging techniques, preventing intragranular cracks and irreversible phase transitions. As a result, IBP-SC92 shows outstanding cycling stability, with nearly 100% capacity retention after 100 cycles in half cells and 94.5% retention after 1,000 cycles in full cells. This redefined single-crystal cathode represents a significant step towards the industrial adoption of high-energy-density materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are included within the article and its Supplementary Information.
References
Liu, T. et al. Understanding Co roles towards developing Co-free Ni-rich cathodes for rechargeable batteries. Nat. Energy 6, 277–286 (2021).
Myung, S.-T. et al. Nickel-rich layered cathode materials for automotive lithium-ion batteries: achievements and perspectives. ACS Energy Lett. 2, 196–223 (2016).
Ryu, H.-H., Park, K.-J., Yoon, C. S. & Sun, Y.-K. Capacity fading of Ni-rich Li[NixCoyMn1–x–y]O2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: bulk or surface degradation. Chem. Mater. 30, 1155–1163 (2018).
Meng, X. H. et al. Kinetic origin of planar gliding in single-crystalline Ni-rich cathodes. J. Am. Chem. Soc. 2022 144, 11338–11347 (2022).
Kim, U.-H. et al. Heuristic solution for achieving long-term cycle stability for Ni-rich layered cathodes at full depth of discharge. Nat. Energy 5, 860–869 (2020).
Li, W., Asl, H. Y., Xie, Q. & Manthiram, A. Collapse of LiNi1−x−yCoxMnyO2 lattice at deep charge irrespective of nickel content in lithium-ion batteries. J. Am. Chem. Soc. 141, 5097–5101 (2019).
Yoon, M. et al. Eutectic salt-assisted planetary centrifugal deagglomeration for single-crystalline cathode synthesis. Nat. Energy 8, 482–491 (2023).
Wang, L., Liu, T., Wu, T. & Lu, J. Strain-retardant coherent perovskite phase stabilized Ni-rich cathode. Nature 611, 61–67 (2022).
Huang, W. et al. Unrecoverable lattice rotation governs structural degradation of single-crystalline cathodes. Science 384, 912–919 (2024).
Ryu, H.-H. et al. Capacity fading mechanisms in Ni-rich single-crystal NCM cathodes. ACS Energy Lett. 6, 2726–2734 (2021).
You, Y., Celio, H., Li, J., Dolocan, A. & Manthiram, A. Modified high-nickel cathodes with stable surface chemistry against ambient air for lithium-ion batteries. Angew. Chem. Int. Ed. Engl. 5, 6480–6485 (2018).
Kim, Y., Park, H., Warner, J. H. & Manthiram, A. Unraveling the intricacies of residual lithium in high-Ni cathodes for lithium-ion batteries. ACS Energy Lett. 6, 941–948 (2021).
Bi, Y. et al. Reversible planar gliding and microcracking in a single-crystalline Ni-rich cathode. Science 370, 1313–1317 (2020).
Hong, Y.-S. et al. Hierarchical defect engineering for LiCoO2 through low-solubility trace element doping. Chem 6, 2759–2769 (2020).
Huang, W. et al. Mechanochemically robust LiCoO2 with ultrahigh capacity and prolonged cyclability. Adv. Mater. 36, 2405519 (2024).
Yan, P. et al. Intragranular cracking as a critical barrier for high-voltage usage of layer-structured cathode for lithium-ion batteries. Nat. Commun. 8, 14101 (2017).
Yu, R. et al. Layer-by-layer delithiation during lattice collapse as the origin of planar gliding and microcracking in Ni-rich cathodes. Cell Rep. Phys. Sci. 4, 7 (2023).
Park, H. et al. In situ multiscale probing of the synthesis of a Ni-rich layered oxide cathode reveals reaction heterogeneity driven by competing kinetic pathways. Nat. Chem. 14, 614–622 (2022).
Qian, G. et al. Single-crystal nickel-rich layered-oxide battery cathode materials: synthesis, electrochemistry, and intra-granular fracture. Energy Storage Mater. 27, 140–149 (2020).
Zhao, W. et al. Quantifying degradation parameters of single-crystalline Ni-rich cathodes in lithium-ion batteries. Angew. Chem. Int. Ed. 62, 202305281 (2023).
Park, K.-J. et al. Improved cycling stability of Li[Ni0.90Co0.05Mn0.05]O2 through microstructure modification by boron doping for Li-ion batteries. Adv. Energy Mater. 8, 1801202 (2018).
Dai, Z., Sun, X., Chen, R., Wu, F. & Li, L. Chemical competing diffusion for practical all-solid-state batteries. J. Am. Chem. Soc. 146, 34517–34527 (2024).
Amalraj, S. F. et al. Boron doped Ni-rich LiNi0.85Co0.10Mn0.05O2 cathode materials studied by structural analysis, solid state NMR, computational modeling, and electrochemical performance. Energy Storage Mater. 42, 594–607 (2021).
Xin, F. et al. What is the role of Nb in nickel-rich layered oxide cathodes for lithium-ion batteries? ACS Energy Lett. 6, 1377–1382 (2021).
Yu, H. et al. Reversible configurations of 3-coordinate and 4-coordinate boron stabilize ultrahigh-Ni cathodes with superior cycling stability for practical Li-ion batteries. Adv. Mater. 37, 2412360 (2024).
Park, G. T. et al. Introducing high-valence elements into cobalt-free layered cathodes for practical lithium-ion batteries. Nat. Energy 7, 946–954 (2022).
Kim, U.-H. et al. High-energy-density Li-ion battery reaching full charge in 12 min. ACS Energy Lett. 7, 3880–3888 (2022).
Chung, H. et al. Mitigating anisotropic changes in classical layered oxide materials by controlled twin boundary defects for long cycle life Li-ion batteries. Chem. Mater. 34, 7302–7312 (2022).
Tang, F. et al. Solute segregation and thermal stability of nanocrystalline solid solution systems. Nanoscale 11, 1813–1826 (2019).
Jiang, Y. et al. Atomistic mechanism of cracking degradation at twin boundary of LiCoO2. Nano Energy 78, 105364 (2020).
Zhang, Q. et al. In situ and real-time monitoring the chemical and thermal evolution of lithium-ion batteries with single-crystalline Ni-rich layered oxide cathode. Angew. Chem. Int. Ed. Engl. 63, e202401716 (2024).
Spence, S. L. et al. Mapping lattice distortions in LiNi0.5Mn1.5O4 cathode materials. ACS Energy Lett. 7, 690–695 (2022).
Zhang, R. et al. Compositionally complex doping for zero-strain zero-cobalt layered cathodes. Nature 610, 67–73 (2022).
Ou, X. et al. Enabling high energy lithium metal batteries via single-crystal Ni-rich cathode material co-doping strategy. Nat. Commun. 13, 2319 (2022).
zhao, C. Suppressing strain propagation in ultrahigh-Ni cathodes during fast charging via epitaxial entropy-assisted coating. Nat. Energy 9, 345–356 (2024).
Hou, X. et al. Lattice oxygen instability in oxide‐based intercalation cathodes: a case study of layered LiNi1/3Co1/3Mn1/3O2. Adv. Energy Mater. 11, 2101005 (2021).
Hu, J. et al. Locking oxygen in lattice: a quantifiable comparison of gas generation in polycrystalline and single crystal Ni-rich cathodes. Energy Storage Mater. 47, 195–202 (2022).
Wang, Y., Zhang, Q., Yang, C. & Xia, Z. Ratiometric fluorescence optical fiber enabling operando temperature monitoring in pouch‐type battery. Adv. Mater. 36, 2401057 (2024).
Mei, W., Jiang, L., Zhou, H., Sun, J. & Wang, Q. Correlating electrochemical performance and heat generation of Li plating for lithium-ion battery with fluoroethylene carbonate additive. J. Energy Chem. 74, 446–453 (2022).
Ahmed, S. et al. Understanding the formation of antiphase boundaries in layered oxide cathode materials and their evolution upon electrochemical cycling. Matter 4, 3953–3966 (2021).
He, D. S. Removal of silicon-containing contaminants from TEM specimens. Ultramicroscopy 253, 113797 (2023).
Milman, V. et al. Electronic structure, properties, and phase stability of inorganic crystals: a pseudopotential plane-wave study. Int. J. Quantum Chem. 77, 895–910 (2000).
Perdew, J. P. et al. Restoring the density-gradient expansion for exchange in solids and surfaces. Phys. Rev. Lett. 100, 136406 (2008).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Acknowledgements
This work was supported by the Major Research plan of the National Natural Science Foundation of China (grant no. 92372207) and the National Key R&D Program of China (grant nos. 2022YFB4000120 and 2023YFB2405800). This work was also supported by Leading Innovative and Entrepreneurial Projects in Zhejiang Province (grant no. 2023R01007). We thank Z. Xia and Y. Wang from the State Key Laboratory of Luminescent Materials and Devices, School of Physics and Optoelectronics at South China University of Technology for their support in providing advanced temperature detection techniques. We thank L. Zeng and Q. Zhang from Southern University of Science and Technology for their assistance in acquiring atomic-resolution STEM data. We acknowledge support from the US Department of Energy (DOE), Office of Energy Efficiency and Renewable Energy, Vehicle Technologies Office. Argonne National Laboratory is operated for DOE Office of Science by UChicago Argonne, LLC, under contract number DE-AC02-06CH11357. Use of the National Synchrotron Light Source II (beamline 3-ID, 7-BM and 18-ID) is supported by the US DOE, an Office of Science user Facility operated by Brookhaven National Laboratory under contract number DE-SC0012704.
Author information
Authors and Affiliations
Contributions
C.Y., Q.Z., T.L., J.W. and J.L. conceived the idea and developed the theory. Q.Z. synthesized the materials and carried out electrochemical testing as well as in situ XRD, XAS and DEMS measurements. W.H., X.H., X.X., T.L., K.A. and L.M. conducted synchrotron XRD, XAS, X-ray scanning nanodiffraction and TXM measurements. Q.Z., J.W. and Y.C. analysed the data. Q.Z., C.Y., J.W. and T.L. wrote the paper. All authors discussed and contributed to the writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Jungjin Park and the other, anonymous, reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–41, Discussion and Tables 1–5.
Supplementary Video 1
The rocking curves of SC92 at the (003) diffraction peak positions.
Supplementary Video 2
The rocking curves of IBP-SC92 at the (003) diffraction peak positions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Q., Wang, J., Chu, Y. et al. Intralattice-bonded phase-engineered ultrahigh-Ni single-crystalline cathodes suppress strain evolution. Nat Energy 10, 1001–1012 (2025). https://doi.org/10.1038/s41560-025-01827-4
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41560-025-01827-4