Abstract
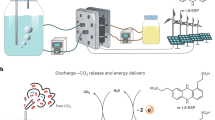
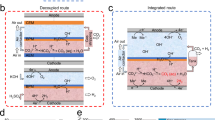
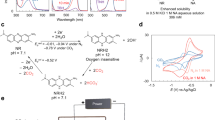
CO2 capture based on a pH swing driven electrically through the reversible proton-coupled electron transfer of organic molecules could be powered entirely by clean electricity. A major technical challenge is the reversible chemical oxidation of the reduced organics by atmospheric O2, which can lower energy efficiency and capture capacity. Here we report the development of a hybrid phenazine flow cell system that uses a pH-swing direct air capture (DAC) process, utilizing redox-active cyclic poly(phenazine sulfide) fabricated solid electrodes. The system maintains a separation between the air and the O2-sensitive reduced phenazine, enabling stable and effective CO2 capture from gas mixtures containing O2. This flow cell demonstrated substantial oxygen compatibility, exhibiting a coulombic efficiency of 99% and requiring only 73 kJ mol−1 CO2 for simulated flue gas and 104 kJ mol−1 CO2 for DAC. The strategy of isolating vulnerable species offers an efficient pathway for DAC and may be broadly applicable to avoiding undesirable side reactions in other electrochemical devices.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
Sanz-Pérez, E. S., Murdock, C. R., Didas, S. A. & Jones, C. W. Direct capture of CO2 from ambient air. Chem. Rev. 116, 11840–11876 (2016).
McQueen, N. et al. A review of direct air capture (DAC): scaling up commercial technologies and innovating for the future. Prog. Energy 3, 032001 (2021).
Lebling, K., Leslie-Bole, H., Byrum, Z. & Bridgwater, L. Direct Air Capture: 6 Things To Know (World Resources Institute, 2022).
Jin, S., Wu, M., Gordon, R. G., Aziz, M. J. & Kwabi, D. G. pH swing cycle for CO2 capture electrochemically driven through proton-coupled electron transfer. Energy Environ. Sci. 13, 3706–3722 (2020).
Sharifian, R., Wagterveld, R. M., Digdaya, I. A., Xiang, C. & Vermaas, D. A. Electrochemical carbon dioxide capture to close the carbon cycle. Energy Environ. Sci. 14, 781–814 (2021).
Renfrew, S. E., Starr, D. E. & Strasser, P. Electrochemical approaches toward CO2 capture and concentration. ACS Catal. 10, 13058–13074 (2020).
Zhu, P. et al. Continuous carbon capture in an electrochemical solid-electrolyte reactor. Nature 618, 959–966 (2023).
Kim, S. et al. Asymmetric chloride-mediated electrochemical process for CO2 removal from oceanwater. Energy Environ. Sci. 16, 2030–2044 (2023).
Li, X., Zhao, X. H., Liu, Y. Y., Hatton, T. A. & Liu, Y. Y. Redox-tunable Lewis bases for electrochemical carbon dioxide capture. Nat. Energy 7, 1065–1075 (2022).
Voskian, S. & Hatton, T. A. Faradaic electro-swing reactive adsorption for CO2 capture. Energy Environ. Sci. 12, 3530–3547 (2019).
Barlow, J. M. et al. Molecular design of redox carriers for electrochemical CO2 capture and concentration. Chem. Soc. Rev. 51, 8415–8433 (2022).
Stern, M. C., Simeon, F., Herzog, H. & Hatton, T. A. Post-combustion carbon dioxide capture using electrochemically mediated amine regeneration. Energy Environ. Sci. 6, 2505–2517 (2013).
Seo, H., Nitzsche, M. P. & Hatton, T. A. Redox-mediated pH swing systems for electrochemical carbon capture. Acc. Chem. Res. 56, 3153–3164 (2023).
Cao, T. N.-D. et al. Unraveling the potential of electrochemical pH-swing processes for carbon dioxide capture and utilization. Ind. Eng. Chem. Res. 62, 20979–20995 (2023).
Jin, S., Wu, M., Jing, Y., Gordon, R. G. & Aziz, M. J. Low-energy carbon capture via electrochemically induced pH swing with electrochemical rebalancing. Nat. Commun. 13, 2140 (2022).
Pang, S. et al. A phenazine-based high-capacity and high-stability electrochemical CO2 capture cell with coupled electricity storage. Nat. Energy 8, 1126–1136 (2023).
Xie, H. et al. Low-energy electrochemical carbon dioxide capture based on a biological redox proton carrier. Cell Rep. Phys. Sci. 1, 100046 (2020).
Xie, H. et al. Low-energy-consumption electrochemical CO2 capture driven by biomimetic phenazine derivatives redox medium. Appl. Energy 259, 114119 (2020).
Seo, H. & Hatton, T. A. Electrochemical direct air capture of CO2 using neutral red as reversible redox-active material. Nat. Commun. 14, 313 (2023).
Seo, H., Rahimi, M. & Hatton, T. A. Electrochemical carbon dioxide capture and release with a redox-active amine. J. Am. Chem. Soc. 144, 2164–2170 (2022).
Zito, A. M., Bím, D., Vargas, S., Alexandrova, A. N. & Yang, J. Y. Computational and experimental design of quinones for electrochemical CO2 capture and concentration. ACS Sustain. Chem. Eng. 10, 11387–11395 (2022).
Bui, A. T., Hartley, N. A., Thom, A. J. W. & Forse, A. C. Trade-off between redox potential and the strength of electrochemical CO2 capture in quinones. J. Phys. Chem. C 126, 14163–14172 (2022).
Lin, K. et al. Alkaline quinone flow battery. Science 349, 1529–1532 (2015).
Pohorecki, R. & Moniuk, W. D. W. Kinetics of reaction between carbon dioxide and hydroxyl ions in aqueous electrolyte solutions. Chem. Eng. Sci. 43, 1677–1684 (1988).
Keith, D. W., Holmes, G., St. Angelo, D. & Heidel, K. A process for capturing CO2 from the atmosphere. Joule 2, 1573–1594 (2018).
Chen, Q., Eisenach, L. & Aziz, M. J. Cycling analysis of a quinone-bromide redox flow battery. J. Electrochem. Soc. 163, a5057–a5063 (2015).
Zuo, P. et al. Near-frictionless ion transport within triazine framework membranes. Nature 617, 299–305 (2023).
Yang, G. et al. Organic electroactive materials for aqueous redox flow batteries. Adv. Mater. 35, e2301898 (2023).
Pan, L. et al. High-performance porous electrodes for flow batteries: improvements of specific surface areas and reaction kinetics. ChemElectroChem 11, e202400460 (2024).
Sun, J. et al. Redox flow batteries and their stack-scale flow fields. Carbon Neutrality 2, 30 (2023).
Zhang, Y. et al. Insights into an air-stable methylene blue catholyte towards kW-scale practical aqueous organic flow batteries. Energy Environ. Sci. 16, 231–240 (2023).
Acknowledgements
We thank Y. Tang from Shanghai Institute of Organic Chemistry for helpful discussions. Financial support received from National Natural Science Foundation of China (grant nos. 22422803, 22101064 and 22375167), the open research fund of Suzhou Laboratory (grant no. SZLAB-1308-2024-TS008), the National Key R&D Program of China (grant nos. 2022YFB2405100 and 2022YFB2405000), the Research Funds of Hangzhou Institute for Advanced Study, UCAS (grant no. 2023HIAS-Y018) and Zhejiang Provincial Natural Science Foundation of China (XHD24B0501) is gratefully acknowledged. Research at Harvard University was supported by the Harvard University Climate Change Solutions Fund. We thank the Instrumentation and Service Center for Physical Science at Westlake University for the facility support and technical assistance.
Author information
Authors and Affiliations
Contributions
Y.J., M.J.A., P.W. and R.G.G. formulated and supervised the project. X.J. and L.L. synthesized the compounds. X.J. performed the CO2 capture tests. S.J. performed the theoretical analysis. S.J., Y.J., P.W. and M.J.A. wrote the paper, and all authors contributed to revising the paper.
Corresponding authors
Ethics declarations
Competing interests
A patent application (CN202410616404.7) has been filed by Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, with Y.J., P.W. and X.J. as inventors.
Peer review
Peer review information
Nature Energy thanks Seoni Kim, Kangkang Li and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–51, Tables 1 and 2 and Notes 1–6.
Source data
Source Data Fig. 3
Source data for the CO2 capture–release system and two typical CO2-concentrating cycles.
Source Data Fig. 4
Source data for the CO2 capture performance at different conditions.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jin, X., Jin, S., Li, L. et al. Direct air capture of CO2 in an electrochemical hybrid flow cell with a spatially isolated phenazine electrode. Nat Energy 10, 1146–1154 (2025). https://doi.org/10.1038/s41560-025-01836-3
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41560-025-01836-3