Abstract
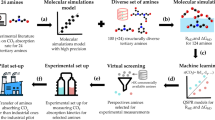
Transforming CO2 into valuable products presents a promising route for reducing emissions across various industry sectors. However, conventional methods, including sequential CO2 electrolysis or reverse water–gas shift reaction, depend on energy-intensive CO2 purification; while emerging reactive CO2 capture strategies still face challenges in designing optimal system components that enable efficient electrochemical regeneration without compromising catalytic performance. Here we systematically screen a broad library of amine-based absorbents to establish a design rationale for tandem amine scrubbing and CO2 electrolysis. We identify piperazine as an optimal capture medium and show that its carbamate form can be directly reduced using a nickel single-atom catalyst. This charge-neutral intermediate facilitates spontaneous adsorption, rapid transport and efficient C–N bond cleavage, enabling stable carbon monoxide production alongside in situ amine regeneration. The process achieves an energy efficiency of ~48.8 GJ per tonne CO, offering a scalable and energy efficient pathway towards carbon-neutral chemical feedstocks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The authors declare that all data supporting the findings of this study are available within the paper, Supplementary Information and Source data files. Source data are provided with this paper.
References
De Luna, P. et al. What would it take for renewably powered electrosynthesis to displace petrochemical processes? Science 364, eaav3506 (2019).
Biddinger, E. J. & Modestino, M. A. Electro-organic syntheses for green chemical manufacturing. Electrochem. Soc. Interface 29, 43 (2020).
Li, M., Irtem, E., Iglesias van Montfort, H.-P., Abdinejad, M. & Burdyny, T. Energy comparison of sequential and integrated CO2 capture and electrochemical conversion. Nat. Commun. 13, 5398 (2022).
González-Castaño, M., Dorneanu, B. & Arellano-García, H. The reverse water gas shift reaction: a process systems engineering perspective. React. Chem. Eng. 6, 954–976 (2021).
Rochelle, G. in Absorption-based Post-Combustion Capture of Carbon Dioxide (ed Feron, P. H. M.) 35–67 (Elsevier: Woodhead Publishing, 2016).
Sullivan, I. et al. Coupling electrochemical CO2 conversion with CO2 capture. Nat. Catal. 4, 952–958 (2021).
Bown, R. M., Joyce, M., Zhang, Q., Reina, T. R. & Duyar, M. S. Identifying commercial opportunities for the reverse water gas shift reaction. Energy Technol. 9, 2100554 (2021).
Heldebrant, D. J., Kothandaraman, J., Dowell, N. M. & Brickett, L. Next steps for solvent-based CO2 capture; integration of capture, conversion, and mineralisation. Chem. Sci. 13, 6445–6456 (2022).
Freyman, M. C. et al. Reactive CO2 capture: a path forward for process integration in carbon management. Joule 7, 631–651 (2023).
Siegel, R. E., Pattanayak, S. & Berben, L. A. Reactive capture of CO2: opportunities and challenges. ACS Catal. 13, 766–784 (2022).
Lee, G. et al. Electrochemical upgrade of CO2 from amine capture solution. Nat. Energy 6, 46–53 (2021).
Conway, W. et al. Designer amines for post combustion CO2 capture processes. Energy Procedia 63, 1827–1834 (2014).
Nakao, S.-I., Yogo, K., Goto, K., Kai, T. & Yamada, H. in Advanced CO2 Capture Technologies: Absorption, Adsorption, and Membrane Separation Methods (ed Hall, C. A. S.) 3–22 (Springer, 2019).
Phillips, R. & Dunnill, C. W. Zero gap alkaline electrolysis cell design for renewable energy storage as hydrogen gas. RSC Adv. 6, 100643–100651 (2016).
Tong, W. et al. Electrolysis of low-grade and saline surface water. Nat. Energy 5, 367–377 (2020).
Yang, Y. et al. Anion-exchange membrane water electrolyzers and fuel cells. Chem. Soc. Rev. 51, 9620–9693 (2022).
Bernhardsen, I. M. & Knuutila, H. K. A review of potential amine solvents for CO2 absorption process: absorption capacity, cyclic capacity and pKa. Int. J. Greenhouse Gas Control 61, 27–48 (2017).
Puxty, G. et al. Carbon dioxide postcombustion capture: a novel screening study of the carbon dioxide absorption performance of 76 amines. Environ. Sci. Technol. 43, 6427–6433 (2009).
El Hadri, N., Quang, D. V., Goetheer, E. L. V. & Abu Zahra, M. R. M. Aqueous amine solution characterization for post-combustion CO2 capture process. Appl. Energy 185, 1433–1449 (2017).
Conway, W. et al. Toward the understanding of chemical absorption processes for post-combustion capture of carbon dioxide: electronic and steric considerations from the kinetics of reactions of CO2(aq) with sterically hindered amines. Environ. Sci. Technol. 47, 1163–1169 (2013).
Yang, Q. et al. A carbon-13 NMR study of carbon dioxide absorption and desorption with aqueous amine solutions. Energy Procedia 1, 955–962 (2009).
Caplow, M. Kinetics of carbamate formation and breakdown. J. Am. Chem. Soc. 90, 6795–6803 (1968).
Johnson, S. & Morrison, D. L. Kinetics and mechanism of decarboxylation of N-arylcarbamates. Evidence for kinetically important zwitterionic carbamic acid species of short lifetime. J. Am. Chem. Soc. 94, 1323–1334 (1972).
da Silva, E. F. & Svendsen, H. F. Ab initio study of the reaction of carbamate formation from CO2 and alkanolamines. Ind. Eng. Chem. Res. 43, 3413–3418 (2004).
Stowe, H. M., Paek, E. & Hwang, G. S. First-principles assessment of CO2 capture mechanisms in aqueous piperazine solution. Phys. Chem. Chem. Phys. 18, 25296–25307 (2016).
Rochelle, G. et al. Aqueous piperazine as the new standard for CO2 capture technology. Chem. Eng. J. 171, 725–733 (2011).
Bishnoi, S. & Rochelle, G. T. Absorption of carbon dioxide into aqueous piperazine: reaction kinetics, mass transfer and solubility. Chem. Eng. Sci. 55, 5531–5543 (2000).
Bishnoi, S. Carbon Dioxide Absorption and Solution Equilibrium in Piperazine-activated Methyldiethanolamine (Univ. of Texas at Austin, 2000).
Freeman, S. A., Dugas, R., Van Wagener, D. H., Nguyen, T. & Rochelle, G. T. Carbon dioxide capture with concentrated, aqueous piperazine. Int. J. Greenhouse Gas Control 4, 119–124 (2010).
Robinson, K., McCluskey, A. & Attalla, M. I. An ATR‐FTIR study on the effect of molecular structural variations on the CO2 absorption characteristics of heterocyclic amines, part II. ChemPhysChem 13, 2331–2341 (2012).
Robinson, K., McCluskey, A. & Attalla, M. I. An FTIR spectroscopic study on the effect of molecular structural variations on the CO2 absorption characteristics of heterocyclic amines. ChemPhysChem 12, 1088–1099 (2011).
Xu, Y. et al. Diffusivity in novel diamine-based water-lean absorbent systems for CO2 capture applications. Ind. Eng. Chem. Res. 61, 12493–12503 (2022).
Leverick, G. et al. Uncovering the active species in amine-mediated CO2 reduction to CO on Ag. ACS Catal. 13, 12322–12337 (2023).
Safipour, J., Weber, A. Z. & Bell, A. T. Detrimental effects of monoethanolamine and other amine-based capture agents on the electrochemical reduction of CO2. ACS Energy Lett. 8, 5012–5017 (2023).
Gu, J., Hsu, C.-S., Bai, L., Chen, H. M. & Hu, X. Atomically dispersed Fe3+ sites catalyze efficient CO2 electroreduction to CO. Science 364, 1091–1094 (2019).
Vijay, S. et al. Unified mechanistic understanding of CO2 reduction to CO on transition metal and single atom catalysts. Nat. Catal. 4, 1024–1031 (2021).
Xia, C. et al. General synthesis of single-atom catalysts with high metal loading using graphene quantum dots. Nat. Chem. 13, 887–894 (2021).
Birdja, Y. Y. et al. Advances and challenges in understanding the electrocatalytic conversion of carbon dioxide to fuels. Nat. Energy 4, 732–745 (2019).
Huang, L. et al. Pressure dependence in aqueous-based electrochemical CO2 reduction. Nat. Commun. 14, 2958 (2023).
Vermaas, D. A. & Smith, W. A. Synergistic electrochemical CO2 reduction and water oxidation with a bipolar membrane. ACS Energy Lett. 1, 1143–1148 (2016).
Sheng, X., Ge, W., Jiang, H. & Li, C. Engineering the Ni-N-C catalyst microenvironment enabling CO2 electroreduction with nearly 100% CO selectivity in acid. Adv. Mater. 34, 2201295 (2022).
Xie, Y. et al. High carbon utilization in CO2 reduction to multi-carbon products in acidic media. Nat. Catal. 5, 564–570 (2022).
Chen, L. et al. Energy-efficient CO(2) conversion to multicarbon products at high rates on CuGa bimetallic catalyst. Nat. Commun. 15, 7053 (2024).
Langie, K. M. G. et al. Toward economical application of carbon capture and utilization technology with near-zero carbon emission. Nat. Commun. 13, 7482 (2022).
Kim, J. H. et al. The insensitive cation effect on a single atom Ni catalyst allows selective electrochemical conversion of captured CO2 in universal media. Energy Environ. Sci. 15, 4301–4312 (2022).
Almajed, H. M. et al. Closing the loop: unexamined performance trade-offs of integrating direct air capture with (bi)carbonate electrolysis. ACS Energy Lett. 9, 2472–2483 (2024).
MacDonald, T. S. C., Price, W. S. & Beves, J. E. Time-resolved diffusion NMR measurements for transient processes. ChemPhysChem 20, 926–930 (2019).
Price, W. S. Pulsed-field gradient nuclear magnetic resonance as a tool for studying translational diffusion: part II. experimental aspects. Concepts Magn. Reson. 10, 197–237 (1998).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Blöchl, P. E., Jepsen, O. & Andersen, O. K. Improved tetrahedron method for Brillouin-zone integrations. Phys. Rev. B 49, 16223–16233 (1994).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. https://doi.org/10.1063/1.3382344 (2010).
Klimeš, J., Bowler, D. R. & Michaelides, A. Van der Waals density functionals applied to solids. Phys. Rev. B https://doi.org/10.1103/PhysRevB.83.195131 (2011).
Di Liberto, G., Cipriano, L. A. & Pacchioni, G. Universal principles for the rational design of single atom electrocatalysts? Handle with care. ACS Catal. 12, 5846–5856 (2022).
Cipriano, L. A., Di Liberto, G. & Pacchioni, G. Superoxo and peroxo complexes on single-atom catalysts: impact on the oxygen evolution reaction. ACS Catal. 12, 11682–11691 (2022).
Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 18, 015012 (2010).
Mao, Y., Wang, Z., Wang, H.-F. & Hu, P. Understanding catalytic reactions over zeolites: a density functional theory study of selective catalytic reduction of NOx by NH3 over Cu-SAPO-34. ACS Catal. 6, 7882–7891 (2016).
Wang, Z., Cao, X. M., Zhu, J. & Hu, P. Activity and coke formation of nickel and nickel carbide in dry reforming: a deactivation scheme from density functional theory. J. Catal. 311, 469–480 (2014).
Nørskov, J. K. et al. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 108, 17886–17892 (2004).
Porezag, D. & Pederson, M. R. Infrared intensities and Raman-scattering activities within density-functional theory. Phys. Rev. B 54, 7830–7836 (1996).
Hjorth Larsen, A. et al. The atomic simulation environment—a Python library for working with atoms. J. Phys. Condens. Matter 29, 273002 (2017).
Frisch, M. J. et al. Gaussian 16 Rev. C.01 (Gaussian, Inc, 2016).
Lee, C., Yang, W. & Parr, R. G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785–789 (1988).
Weigend, F. Accurate Coulomb-fitting basis sets for H to Rn. Phys. Chem. Chem. Phys. 8, 1057–1065 (2006).
Weigend, F. & Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys. Chem. Chem. Phys. 7, 3297–3305 (2005).
Marenich, A. V., Cramer, C. J. & Truhlar, D. G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 113, 6378–6396 (2009).
Renon, H. & Prausnitz, J. M. Local compositions in thermodynamic excess functions for liquid mixtures. AlChE J. 14, 135–144 (1968).
Frailie, P., Plaza, J., Van Wagener, D. & Rochelle, G. T. Modeling piperazine thermodynamics. Energy Procedia 4, 35–42 (2011).
Du, Y. & Rochelle, G. T. Thermodynamic modeling of aqueous piperazine/N-(2-aminoethyl) piperazine for CO2 capture. Energy Procedia 63, 997–1017 (2014).
Yan, Y. & Chen, C.-C. Thermodynamic modeling of CO2 solubility in aqueous solutions of NaCl and Na2SO4. J. Supercrit. Fluids 55, 623–634 (2010).
Hamborg, E. S. & Versteeg, G. F. Dissociation constants and thermodynamic properties of amines and alkanolamines from (293 to 353) K. J. Chem. Eng. Data 54, 1318–1328 (2009).
Hilliard, M. D. A Predictive Thermodynamic Model for an Aqueous Blend of Potassium Carbonate, Piperazine, and Monoethanolamine for Carbon Dioxide Capture from Flue Gas (Univ. of Texas at Austin, 2008).
Kamps, Á. P.-S., Xia, J. & Maurer, G. Solubility of CO2 in (H2O+piperazine) and in (H2O+MDEA+piperazine). AlChE J. 49, 2662–2670 (2003).
Ermatchkov, V., Pérez-Salado Kamps, Á., Speyer, D. & Maurer, G. Solubility of carbon dioxide in aqueous solutions of piperazine in the low gas loading region. J. Chem. Eng. Data 51, 1788–1796 (2006).
Dugas, R. E. Carbon Dioxide Absorption, Desorption, and Diffusion in Aqueous Piperazine and Monoethanolamine (Univ. of Texas at Austin, 2009).
Xu, Q. & Rochelle, G. Total pressure and CO2 solubility at high temperature in aqueous amines. Energy Procedia 4, 117–124 (2011).
Ermatchkov, V., Pérez-Salado Kamps, Á. & Maurer, G. Chemical equilibrium constants for the formation of carbamates in (carbon dioxide+piperazine+water) from 1H-NMR-spectroscopy. J. Chem. Thermodyn. 35, 1277–1289 (2003).
Gu, J. et al. Modulating electric field distribution by alkali cations for CO2 electroreduction in strongly acidic medium. Nat. Catal. 5, 268–276 (2022).
Acknowledgements
We would like to thank the financial support from the Australian Research Council (ARC) through Future Fellowship (FT210100298, FT210100806), DECRA (DE230101068), Discovery Project (DP220100603), Linkage Project (LP220100088, LP210200504, LP210200345, LP210100467, LP230200897), Industrial Transformation Training Centre (IC180100005) and Industrial Transformation Research Hub (IH240100009) schemes, the Australian Government through the Cooperative Research Centres Projects (CRCPXIII000077) and the Australian Renewable Energy Agency (ARENA) as part of ARENA’s Transformative Research Accelerating Commercialization Program (TM021) and European Commission’s Australia-Spain Network for Innovation and Research Excellence (AuSpire). P.L. would like to thank Australian Nuclear Science and Technology Organisation (ANSTO) for providing beamline access and technical support to complete X-ray absorption spectroscopy (XAS) tests at the Australian Synchrotron via the Medium Energy X-ray (MEX) beamline (beamtime: M20409/M21349). We acknowledge the use of the instruments and scientific and technical assistance at RMIT Microscopy and Microanalysis Facility (RMMF) and the Monash Centre for Electron Microscopy, a Node of Microscopy Australia (ARC LE0454166). The computational study is supported by the Marsden Fund Council from Government funding (21–UOA–237) and Catalyst: Seeding General Grant (22–UOA–031–CGS), managed by Royal Society Te Apārangi. Z.W. and Y.M. acknowledge the use of New Zealand eScience Infrastructure (NeSI) high-performance computing facilities, consulting support and/or training services as part of this research. G.I.N.W. and Y.M. acknowledge funding support from the MacDiarmid Institute for Advanced Materials and Nanotechnology, the Energy Education Trust of New Zealand and the Royal Society Te Apārangi.
Author information
Authors and Affiliations
Contributions
P.L. and T.M. conceived the idea, designed the study, prepared the catalysts and conducted catalyst characterizations and electrochemical tests. H.S. and P.L. conducted full-cell electrolyser tests. Y.M. and Z.W. conducted the DFT calculations and Gaussian simulations. X.C. did the electron microscopy characterizations. H.J. H.Y. and W.K.P. collected the FTIR spectra data. Q.Y., K.L. and K.H. conducted the 13C NMR test. R.M. contributed to the diffusion tests. Z.Z., Y.Z. and E.F. did the XAS test. Y.L., B.J. and G.I.N.W. discussed the electrochemical data.
Corresponding authors
Ethics declarations
Competing interests
P.L. and T.M. are listed as inventors on a patent application filed by RMIT University that pertains to this work (PCT/AU2025/050293). The other authors declare no competing interests.
Peer review
Peer review information
Nature Energy thanks Federico Dattila and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Notes 1–12, Figs. 1–75, Tables 1–19 and Reference.
Supplementary Video
Simulated IR spectra from DFT calculations and the animations of corresponding vibration modes.
Supplementary Source Data
Additional supplementary source data.
Source data
Source Data Fig. 2
Unprocessed source data.
Source Data Fig. 3
Unprocessed source data.
Source Data Fig. 4
Unprocessed source data.
Source Data Fig. 5
Unprocessed source data.
Source Data Fig. 6
Unprocessed source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, P., Mao, Y., Shin, H. et al. Tandem amine scrubbing and CO2 electrolysis via direct piperazine carbamate reduction. Nat Energy 10, 1262–1273 (2025). https://doi.org/10.1038/s41560-025-01869-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41560-025-01869-8
This article is cited by
-
Diverging maturity and converging challenges of water and CO₂ electrolysis in 2025
Nature Reviews Clean Technology (2026)
-
Binding and release in balance
Nature Energy (2025)