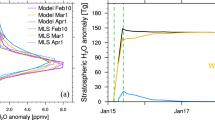
Abstract
The explosive January 2022 Hunga submarine eruption in Tonga injected unprecedented water volumes into the upper atmosphere, generating widespread climatic impacts. However, it ejected anomalously little sulfur compared with other eruptions of similar volume. We explain the missing sulfur with volatile budgets calculated from volcanic ash samples spanning the eruption. We show that magma was stored in a weakly stratified reservoir at 2.1 km to >5.6 km depth. Magma rose within <3 min and fragmented at 400–1,000 m below sea level. This preserves microscale chemical mingling including ~1 wt% contrasts in magmatic water concentrations. The 11-h eruption released a total of 319 Tg of magmatic water, which is <10% of that derived from magmatic seawater interaction. Comparing magmatic and residual glass sulfur concentrations shows a total release of 9.4 TgS, but >93% of this entered the ocean during submarine magma fragmentation. These results raise the concern that satellite SO2 monitoring underestimates the magma output of submarine explosions and they are probably near invisible in ice-core records, despite their climate influence caused by water injection into the upper atmosphere.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data for this paper are provided in Supplementary Tables 1–6 and are available via Figshare at https://doi.org/10.6084/m9.figshare.28665107 (ref. 68).
References
Roggensack, K., Williams, S. N., Schaefer, S. J. & Parnell, R. A. Jr Volatiles from the 1994 eruptions of Rabaul: understanding large caldera systems. Science 273, 490–493 (1996).
Wallace, P. J., Plank, T., Edmonds, M. & Hauri, E. H. in Encyclopedia of Volcanoes 163–183 (Elsevier, 2015).
Kelly, P. M. & Sear, C. B. Climatic impact of explosive volcanic eruptions. Nature 311, 740–743 (1984).
Hansen, J., Lacis, A., Ruedy, R. & Sato, M. Potential climate impact of Mount Pinatubo eruption. Geophys. Res. Lett. 19, 215–218 (1992).
Matoza, R. S. et al. Atmospheric waves and global seismoacoustic observations of the January 2022 Hunga eruption, Tonga. Science 377, 95–100 (2022).
Proud, S. R., Prata, A. T. & Schmauß, S. The January 2022 eruption of Hunga Tonga-Hunga Ha’apai volcano reached the mesosphere. Science 378, 554–557 (2022).
Cronin, S. J. et al. Extreme explosivity of the 15 January 2022 Hunga eruption, Tonga, driven by magma-mixing, caldera collapse and magma-water interaction. In IAVCEI Conference 1414 (IAVCEI, 2023).
Carn, S., Krotkov, N., Fisher, B. & Li, C. Out of the blue: volcanic SO2 emissions during the 2021–2022 eruptions of Hunga Tonga—Hunga Ha’apai (Tonga). Front. Earth Sci. 10, 976962 (2022).
Guo, S., Rose, W. I., Bluth, G. J. & Watson, I. M. Particles in the great Pinatubo volcanic cloud of June 1991: the role of ice. Geochem. Geophys. Geosyst. 5, Q05003 (2004).
Mastin, L. G., Van Eaton, A. R. & Cronin, S. J. Did steam boost the height and growth of the giant Hunga eruption plume? Bull. Volcanol. 86, 64 (2024).
Millan, L. et al. The Hunga Tonga-Hunga Ha’apai hydration of the stratosphere. Geophys. Res. Lett. 49, e2022GL099381 (2022).
Vömel, H., Evan, S. & Tully, M. Water vapor injection into the stratosphere by Hunga Tonga-Hunga Ha’apai. Science 377, 1444–1447 (2022).
Wilmouth, D. M., Østerstrøm, F. F., Smith, J. B., Anderson, J. G. & Salawitch, R. J. Impact of the Hunga Tonga volcanic eruption on stratospheric composition. Proc. Natl Acad. Sci. USA 120, e2301994120 (2023).
Evan, S. et al. Rapid ozone depletion after humidification of the stratosphere by the Hunga Tonga eruption. Science 382, eadg2551 (2023).
Colombier, M. et al. Atmosphere injection of sea salts during large explosive submarine volcanic eruptions. Sci. Rep. 13, 14435 (2023).
Schellart, W., Lister, G. & Toy, V. A Late Cretaceous and Cenozoic reconstruction of the Southwest Pacific region: tectonics controlled by subduction and slab rollback processes. Earth Sci. Rev. 76, 191–233 (2006).
Brenna, M. et al. Post-caldera volcanism reveals shallow priming of an intra-ocean arc andesitic caldera: Hunga volcano, Tonga, SW Pacific. Lithos 412, 106614 (2022).
Cronin, S. et al. New volcanic island unveils explosive past. Eos 98, 18–23 (2017).
Lynett, P. et al. Diverse tsunamigenesis triggered by the Hunga Tonga-Hunga Ha’apai eruption. Nature 609, 728–733 (2022).
Clare, M. A. et al. Fast and destructive density currents created by ocean-entering volcanic eruptions. Science 381, 1085–1092 (2023).
Borrero, J. C. et al. Tsunami runup and inundation in Tonga from the January 2022 eruption of Hunga volcano. Pure Appl. Geophys. 180, 1–22 (2023).
Purkis, S. J. et al. The 2022 Hunga-Tonga megatsunami: near-field simulation of a once-in-a-century event. Sci. Adv. 9, eadf5493 (2023).
Le Mével, H., Miller, C. A., Ribó, M., Cronin, S. & Kula, T. The magmatic system under Hunga volcano before and after the 15 January 2022 eruption. Sci. Adv. 9, eadh3156 (2023).
Stolper, E. The speciation of water in silicate melts. Geochim. Cosmochim. Acta 46, 2609–2620 (1982).
Caulfield, J., Cronin, S. J., Turner, S. P. & Cooper, L. B. Mafic Plinian volcanism and ignimbrite emplacement at Tofua volcano, Tonga. Bull. Volcanol. 73, 1259–1277 (2011).
Cooper, L., Plank, T., Arculus, R., Hauri, E. & Kelley, K. A. Arc–backarc exchange along the Tonga–Lau system: constraints from volatile elements. J. Petrol. 63, egac072 (2022).
Moore, L. R. et al. Bubbles matter: an assessment of the contribution of vapor bubbles to melt inclusion volatile budgets. Am. Miner. 100, 806–823 (2015).
Firth, C. et al. Experimental constraints on the differentiation of low-alkali magmas beneath the Tonga arc: implications for the origin of arc tholeiites. Lithos 344, 440–451 (2019).
Behrens, H., Zhang, Y. & Xu, Z. H2O diffusion in dacitic and andesitic melts. Geochim. Cosmochim. Acta 68, 5139–5150 (2004).
Kelly, L. J. et al. Airfall volume of the 15 January 2022 eruption of Hunga volcano estimated from ocean color changes. Bull. Volcanol. 86, 59 (2024).
Paredes-Mariño, J. et al. Fragmentation mechanisms of the 2022 Hunga eruption inferred from volcanic ash properties from land and sea. In AGU23 Conference V43A-03 (AGU, 2023).
Keppler, H. Experimental evidence for the source of excess sulfur in explosive volcanic eruptions. Science 284, 1652–1654 (1999).
Parmigiani, A., Faroughi, S., Huber, C., Bachmann, O. & Su, Y. Bubble accumulation and its role in the evolution of magma reservoirs in the upper crust. Nature 532, 492–495 (2016).
Muth, M. J. & Wallace, P. J. Sulfur recycling in subduction zones and the oxygen fugacity of mafic arc magmas. Earth Planet. Sci. Lett. 599, 117836 (2022).
Rutherford, M. J. & Devine, J. D. in Fire and Mud: Eruptions and Lahars of Mount Pinatubo, Philippines (eds Newhall, C.G. & Punongbayan, R.S.) 751–766 (University of Washington Press, 1996).
Caulfield, J., Turner, S. P., Smith, I., Cooper, L. & Jenner, G. A. Magma evolution in the primitive, intra-oceanic Tonga arc: petrogenesis of basaltic andesites at Tofua volcano. J. Petrol. 53, 1197–1230 (2012).
Scaillet, B., Clémente, B., Evans, B. W. & Pichavant, M. Redox control of sulfur degassing in silicic magmas. J. Geophys. Res. Solid Earth 103, 23937–23949 (1998).
Deering, C. D., Gravley, D. M., Vogel, T. A., Cole, J. W. & Leonard, G. S. Origins of cold-wet-oxidizing to hot-dry-reducing rhyolite magma cycles and distribution in the Taupo volcanic zone, New Zealand. Contrib. Mineral. Petrol. 160, 609–629 (2010).
Cadoux, A., Scaillet, B., Druitt, T. H. & Deloule, E. Magma storage conditions of large Plinian eruptions of Santorini Volcano (Greece). J. Petrol. 55, 1129–1171 (2014).
Plank, T., Kelley, K. A., Zimmer, M. M., Hauri, E. H. & Wallace, P. J. Why do mafic arc magmas contain ∼4 wt% water on average? Earth Planet. Sci. Lett. 364, 168–179 (2013).
Mellors, R. & Sparks, R. Spatter-rich pyroclastic flow deposits on Santorini, Greece. Bull. Volcanol. 53, 327–342 (1991).
Firth, C. W. et al. Dynamics and pre-eruptive conditions of catastrophic, ignimbrite-producing eruptions from the Yenkahe Caldera, Vanuatu. J. Volcanol. Geotherm. Res. 308, 39–60 (2015).
Jorgenson, C., Caricchi, L., Chiaradia, M., Agreda-López, M. & Giordano, G. Rapid accumulation and ascent precedes caldera forming eruption of low viscosity magma. Contrib. Mineral. Petrol. 179, 16 (2024).
Sigl, M. et al. Timing and climate forcing of volcanic eruptions for the past 2,500 years. Nature 523, 543–549 (2015).
Sigl, M., Toohey, M., McConnell, J. R., Cole-Dai, J. & Severi, M. Volcanic stratospheric sulfur injections and aerosol optical depth during the Holocene (past 11500 years) from a bipolar ice-core array. Earth Syst. Sci. Data 14, 3167–3196 (2022).
McIntosh, I. M., Nichols, A. R., Tani, K. & Llewellin, E. W. Accounting for the species-dependence of the 3500 cm−1 H2Ot infrared molar absorptivity coefficient: implications for hydrated volcanic glasses. Am. Miner. 102, 1677–1689 (2017).
Mandeville, C. W. et al. Determination of molar absorptivities for infrared absorption bands of H2O in andesitic glasses. Am. Miner. 87, 813–821 (2002).
Wysoczanski, R. & Tani, K. Spectroscopic FTIR imaging of water species in silicic volcanic glasses and melt inclusions: an example from the Izu-Bonin arc. J. Volcanol. Geotherm. Res. 156, 302–314 (2006).
Nichols, A. R. & Wysoczanski, R. Using micro-FTIR spectroscopy to measure volatile contents in small and unexposed inclusions hosted in olivine crystals. Chem. Geol. 242, 371–384 (2007).
Tollan, P., Ellis, B., Troch, J. & Neukampf, J. Assessing magmatic volatile equilibria through FTIR spectroscopy of unexposed melt inclusions and their host quartz: a new technique and application to the Mesa Falls Tuff, Yellowstone. Contrib. Mineral. Petrol. 174, 24 (2019).
Le Losq, C., Neuville, D. R., Moretti, R. & Roux, J. Determination of water content in silicate glasses using Raman spectrometry: implications for the study of explosive volcanism. Am. Miner. 97, 779–790 (2012).
Long, D. A. Raman Spectroscopy (McGraw-Hill, 1977).
van Gerve, T. D. & Namur, O. SilicH2O: a graphical user interface for processing silicate glass Raman spectra and quantifying H2O. Volcanica 6, 405–413 (2023).
González-García, D. et al. Water-enhanced interdiffusion of major elements between natural shoshonite and high-K rhyolite melts. Chem. Geol. 466, 86–101 (2017).
Nandedkar, R. H., Ulmer, P. & Müntener, O. Fractional crystallization of primitive, hydrous arc magmas: an experimental study at 0.7 GPa. Contrib. Mineral. Petrol. 167, 1015 (2014).
Almeev, R. R., Holtz, F., Ariskin, A. A. & Kimura, J.-I. Storage conditions of Bezymianny volcano parental magmas: results of phase equilibria experiments at 100 and 700 MPa. Contrib. Mineral. Petrol. 166, 1389–1414 (2013).
Putirka, K. D. Thermometers and barometers for volcanic systems. Rev. Mineral. Geochem. 69, 61–120 (2008).
Kress, V. C. & Carmichael, I. S. The compressibility of silicate liquids containing Fe2O3 and the effect of composition, temperature, oxygen fugacity and pressure on their redox states. Contrib. Mineral. Petrol. 108, 82–92 (1991).
Mollo, S., Putirka, K., Misiti, V., Soligo, M. & Scarlato, P. A new test for equilibrium based on clinopyroxene–melt pairs: clues on the solidification temperatures of Etnean alkaline melts at post-eruptive conditions. Chem. Geol. 352, 92–100 (2013).
Mollo, S. & Masotta, M. Optimizing pre-eruptive temperature estimates in thermally and chemically zoned magma chambers. Chem. Geol. 368, 97–103 (2014).
Lindsley, D. H. Pyroxene thermometry. Am. Miner. 68, 477–493 (1983).
Droop, G. A general equation for estimating Fe3+ concentrations in ferromagnesian silicates and oxides from microprobe analyses, using stoichiometric criteria. Mineral. Mag. 51, 431–435 (1987).
Putirka, K. D. Igneous thermometers and barometers based on plagioclase + liquid equilibria: tests of some existing models and new calibrations. Am. Miner. 90, 336–346 (2005).
Lange, R. A., Frey, H. M. & Hector, J. A thermodynamic model for the plagioclase-liquid hygrometer/thermometer. Am. Miner. 94, 494–506 (2009).
Iacovino, K., Matthews, S., Wieser, P. E., Moore, G. & Bégué, F. VESIcal part I: an open-source thermodynamic model engine for mixed volatile (H2O-CO2) solubility in silicate melts. Earth Space Sci. 8, e2020EA001584 (2021).
Ghiorso, M. S. & Gualda, G. A. An H2O-CO2 mixed fluid saturation model compatible with rhyolite-MELTS. Contrib. Mineral. Petrol. 169, 53 (2015).
Iacovino, K. & Till, C. B. DensityX: a program for calculating the densities of magmatic liquids up to 1,627 °C and 30 kbar. Volcanica 2, 1–10 (2019).
Wu, J. et al. Data for ‘Low sulfur emissions from 2022 Hunga eruption due to seawater-magma interactions’. Figshare https://doi.org/10.6084/m9.figshare.28665107 (2025).
Acknowledgements
R. Almeev and F. Marxer (Leibniz University Hannover) are acknowledged for providing Raman glass standards. This research is financially supported by New Zealand Government Ministry of Business (Innovation and Employment Endeavour Research Program UOA24103), Royal Society of New Zealand Te Apārangi (Marsden project MFP-UOO2218), Australian Nuclear Science and Technology Organisation (ANSTO), Australian Synchrotron and the New Zealand Synchrotron Group (project M18638), the Korean Polar Research Institute (project PE22550) and the Alexander von Humboldt Foundation (for D.G.-G.).
Author information
Authors and Affiliations
Contributions
Conceptualization: S.J.C.; methodology: J.W., S.J.C., I.A.U.; sample collection: S.J.C., F.L., T.K., S.-H.P.; data collection: J.W., S.J.C., I.A.U., D.A., J.P.-M., K.H., M.H., D.G.-G., A.R.L.N., J.V., A.K.; formal analysis: J.W., S.J.C., M.B.; funding acquisition: S.J.C., M.B., S.-H.P.; supervision: S.J.C., M.B.; writing–original draft: J.W., S.J.C., M.B.; writing–review and editing: all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Philipson Bani, Razvan Popa and Thor Hansteen for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Microscopic images of melt inclusions in different host mineral phases.
Mineral hosts include olivine, orthopyroxene, clinopyroxene and plagioclase.
Extended Data Fig. 2 Compositional variations with changing Mg# in melt inclusions and matrix glass associated to different mineral phases from Hunga samples.
a, SiO2. b, CaO. c, Na2O. d, K2O. e, Al2O3. f, TiO2. g, P2O5. h, Cl. Mg# = molar Mg/(Mg + Fe2+) × 100.
Extended Data Fig. 3 Plot of the ratio between molecular water (H2Om) and hydroxyl (OH) vs total water (H2Ot) in matrix glass from Hunga pyroclasts.
The density of overlapping data points are indicated by the colour code for n = 812 analyses.
Extended Data Fig. 4 Frequency histogram of S concentrations in microlite-poor matrix glass from Hunga.
a, Subaerial samples covering the whole 2022 Hunga eruptive stages (n = 1,112). b, Submarine samples (n = 168). Kernel density estimate (KDE) curves are shown. Below detection limit (~30 ppm S) concentrations were found for 186 analyses in a and for 30 in b.
Extended Data Fig. 5 Frequency histogram of Raman H2O concentrations in microlite-poor matrix glass from Hunga.
a, Subaerial samples covering the whole 2022 Hunga eruptive stages (n = 161). b, Submarine samples (n = 56). KDE curves are shown. Below detection limit (~0.1 wt% H2O) concentrations were found for nine analyses each in a and b.
Extended Data Fig. 6 Calibration line for quantification of H2O concentration by Raman spectroscopy (n = 14).
Error bars represent 2 SD uncertainty of several measurements of Rw/s by Raman (n = 3–4) and H2O concentration by Karl Fischer Titration (KFT, n = 2) and/or FTIR (n = 4). Some error bars are smaller than the plot symbol. \({C}_{{{\rm{H}}}_{2}{\rm{O}}}\) is given in wt% (Methods).
Extended Data Fig. 7 Measured vs predicted clinopyroxene DiHd components for Hunga samples.
Predicted values calculated from post-entrapment crystallization (PEC) corrected melt inclusions.
Supplementary information
Supplementary Tables
Six supplementary data tables including major element and volatile compositions for Hunga pyroclasts and standards.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, J., Cronin, S.J., Brenna, M. et al. Low sulfur emissions from 2022 Hunga eruption due to seawater–magma interactions. Nat. Geosci. 18, 518–524 (2025). https://doi.org/10.1038/s41561-025-01691-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41561-025-01691-7