Abstract
Forest soils hold the largest terrestrial carbon pool, derived from dead plant tissues and transformed by soil biota. Current frameworks emphasize the role of soil microbes in highly persistent forms of carbon. However, moderately persistent forms of carbon also contribute substantially to forest soil carbon pools through the iterative effects of plant litter inputs and outputs over multi-decadal timescales. These sources of soil carbon are not well constrained. Here we synthesize published field data of the finest roots (absorptive roots) of mycorrhizal woody plants across major forest ecosystem types in the Northern Hemisphere. We estimate that, owing to fast turnover and slow decomposition, the iterative effects of absorptive roots on soil carbon accrual generate 2.4 ± 0.1 MgC ha−1 over two decades, exceeding that of leaves by 65%. Further, roots associated with arbuscular mycorrhizal fungi contribute 43% more soil carbon than roots associated with ectomycorrhizal fungi, despite ectomycorrhizal forests dominating soil carbon storage in forest soils overall. We also find that specific root length, a readily measured trait, can be used as a proxy for iterative effects associated with root dynamics. Our findings thus provide a long-needed belowground metric for carbon modelling in the Earth system.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data used in this study are available via figshare at https://doi.org/10.6084/m9.figshare.26995555.v3 (ref. 79).
Code availability
All R code used in the analyses is available via figshare at https://doi.org/10.6084/m9.figshare.26995555.v3 (ref. 79).
References
Pan, Y. et al. A large and persistent carbon sink in the world’s forests. Science 333, 988–993 (2011).
Whalen, E. D. et al. Clarifying the evidence for microbial‐ and plant‐derived soil organic matter, and the path toward a more quantitative understanding. Glob. Change Biol. 28, 7167–7185 (2022).
Liang, C., Amelung, W., Lehmann, J. & Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 25, 3578–3590 (2019).
Tao, F. et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 618, 981–985 (2023).
Derrien, D. et al. Current controversies on mechanisms controlling soil carbon storage: implications for interactions with practitioners and policy-makers. A review. Agron. Sustain. Dev. 43, 21 (2023).
Sokol, N. W. et al. Global distribution, formation and fate of mineral‐associated soil organic matter under a changing climate: a trait‐based perspective. Funct. Ecol. 36, 1411–1429 (2022).
Keiluweit, M. et al. Mineral protection of soil carbon counteracted by root exudates. Nat. Clim. Change 5, 588–595 (2015).
Liu, M. et al. Unprotected carbon dominates decadal soil carbon increase. Nat. Commun. 16, 2008 (2025).
Angst, G. et al. Unlocking complex soil systems as carbon sinks: multi-pool management as the key. Nat. Commun. 14, 2967 (2023).
Kou, L. et al. Nitrogen deposition increases root production and turnover but slows root decomposition in Pinus elliottii plantations. N. Phytol. 218, 1450–1461 (2018).
De Deyn, G. B., Cornelissen, J. H. C. & Bardgett, R. D. Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 11, 516–531 (2008).
Clemmensen, K. E. et al. Roots and associated fungi drive long-term carbon sequestration in boreal forest. Science 339, 1615–1618 (2013).
McCormack, M. L., Adams, T. S., Smithwick, E. A. H. & Eissenstat, D. M. Predicting fine root lifespan from plant functional traits in temperate trees. N. Phytol. 195, 823–831 (2012).
Panchal, P., Preece, C., Peñuelas, J. & Giri, J. Soil carbon sequestration by root exudates. Trends Plant Sci. 27, 749–757 (2022).
Jiang, L., Kou, L. & Li, S. Alterations of early-stage decomposition of leaves and absorptive roots by deposition of nitrogen and phosphorus have contrasting mechanisms. Soil Biol. Biochem. 127, 213–222 (2018).
Sun, T. et al. Contrasting dynamics and trait controls in first-order root compared with leaf litter decomposition. Proc. Natl Acad. Sci. USA 115, 10392–10397 (2018).
McCormack, M. L. et al. Redefining fine roots improves understanding of below‐ground contributions to terrestrial biosphere processes. N. Phytol. 207, 505–518 (2015).
Kou, L., Ma, N., Freschet, G. T., McCormack, M. L. & Li, S. Iterative effects: a new paradigm for root dynamics. Trends Plant Sci. 30, 941–952 (2025).
See, C. R. et al. Global patterns in fine root decomposition: climate, chemistry, mycorrhizal association and woodiness. Ecol. Lett. 22, 946–953 (2019).
Hou, J. et al. Linking fine root lifespan to root chemical and morphological traits—a global analysis. Proc. Natl Acad. Sci. USA 121, e2320623121 (2024).
Norby, R. J. & Jackson, R. B. Root dynamics and global change: seeking an ecosystem perspective. N. Phytol. 147, 3–12 (2000).
Soudzilovskaia, N. A. et al. Global mycorrhizal plant distribution linked to terrestrial carbon stocks. Nat. Commun. 10, 5077 (2019).
Phillips, R. P., Brzostek, E. & Midgley, M. G. The mycorrhizal‐associated nutrient economy: a new framework for predicting carbon–nutrient couplings in temperate forests. N. Phytol. 199, 41–51 (2013).
Zheng, J. J. et al. A trait-based root acquisition-defence-decomposition framework in angiosperm tree species. Nat. Commun. 15, 5311 (2024).
Bardgett, R. D., Mommer, L. & De Vries, F. T. Going underground: root traits as drivers of ecosystem processes. Trends Ecol. Evol. 29, 692–699 (2014).
Weemstra, M. et al. Towards a multidimensional root trait framework: a tree root review. N. Phytol. 211, 1159–1169 (2016).
Guo, D. L., Mitchell, R. J. & Hendricks, J. J. Fine root branch orders respond differentially to carbon source–sink manipulations in a longleaf pine forest. Oecologia 140, 450–457 (2004).
Bergmann, J. et al. The fungal collaboration gradient dominates the root economics space in plants. Sci. Adv. 6, eaba3756 (2020).
Ma, H. et al. The global distribution and environmental drivers of aboveground versus belowground plant biomass. Nat. Ecol. Evol. 5, 1110–1122 (2021).
Xiao, L. et al. Global depth distribution of belowground net primary productivity and its drivers. Glob. Ecol. Biogeogr. 32, 1435–1451 (2023).
Olson, J. S. Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44, 322–331 (1963).
Lavallee, J. M., Soong, J. L. & Cotrufo, M. F. Conceptualizing soil organic matter into particulate and mineral‐associated forms to address global change in the 21st century. Glob. Change Biol. 26, 261–273 (2020).
Fan, X. et al. Improved model simulation of soil carbon cycling by representing the microbially derived organic carbon pool. ISME J. 15, 2248–2263 (2021).
Gao, D., Bai, E., Wasner, D. & Hagedorn, F. Global prediction of soil microbial growth rates and carbon use efficiency based on the metabolic theory of ecology. Soil Biol. Biochem. 190, 109315 (2024).
Reich, P. B. et al. Temperature drives global patterns in forest biomass distribution in leaves, stems, and roots. Proc. Natl Acad. Sci. USA 111, 13721–13726 (2014).
Gill, R. A. & Jackson, R. B. Global patterns of root turnover for terrestrial ecosystems. N. Phytol. 147, 13–31 (2000).
Steidinger, B. S. et al. Climatic controls of decomposition drive the global biogeography of forest-tree symbioses. Nature 569, 404–408 (2019).
Averill, C., Turner, B. L. & Finzi, A. C. Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature 505, 543–545 (2014).
Zhu, K., McCormack, M. L., Lankau, R. A., Egan, J. F. & Wurzburger, N. Association of ectomycorrhizal trees with high carbon‐to‐nitrogen ratio soils across temperate forests is driven by smaller nitrogen not larger carbon stocks. J. Ecol. 106, 524–535 (2018).
Keller, A. B., Brzostek, E. R., Craig, M. E., Fisher, J. B. & Phillips, R. P. Root‐derived inputs are major contributors to soil carbon in temperate forests, but vary by mycorrhizal type. Ecol. Lett. 24, 626–635 (2021).
Craig, M. E. et al. Tree mycorrhizal type predicts within‐site variability in the storage and distribution of soil organic matter. Glob. Change Biol. 24, 3317–3330 (2018).
Liang, C., Schimel, J. P. & Jastrow, J. D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2, 17105 (2017).
Frey, S. D. Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 50, 237–259 (2019).
Zhang, Z. et al. Mycelia‐derived C contributes more to nitrogen cycling than root‐derived C in ectomycorrhizal alpine forests. Funct. Ecol. 33, 346–359 (2019).
Jia, Y. et al. Soil organic carbon sourcing variance in the rhizosphere vs. non-rhizosphere of two mycorrhizal tree species. Soil Biol. Biochem. 176, 108884 (2023).
Gadgil, R. L. & Gadgil, P. D. Mycorrhiza and litter decomposition. Nature 233, 133–133 (1971).
Reich, P. B. The world-wide ‘fast–slow’ plant economics spectrum: a traits manifesto. J. Ecol. 102, 275–301 (2014).
Weemstra, M. et al. The role of fine‐root mass, specific root length and life span in tree performance: a whole‐tree exploration. Funct. Ecol. 34, 575–585 (2020).
Ostonen, I. et al. Specific root length as an indicator of environmental change. Plant Biosyst. 141, 426–442 (2007).
Hobbie, S. E., Oleksyn, J., Eissenstat, D. M. & Reich, P. B. Fine root decomposition rates do not mirror those of leaf litter among temperate tree species. Oecologia 162, 505–513 (2010).
Iversen, C. M. et al. A global fine‐root ecology database to address below‐ground challenges in plant ecology. N. Phytol. 215, 15–26 (2017).
Guo, L. et al. The coordination between leaf and fine root litter decomposition and the difference in their controlling factors. Glob. Ecol. Biogeogr. 30, 2286–2296 (2021).
Wang, J., Tharayil, N., Chow, A. T., Suseela, V. & Zeng, H. Phenolic profile within the fine‐root branching orders of an evergreen species highlights a disconnect in root tissue quality predicted by elemental‐ and molecular‐level carbon composition. N. Phytol. 206, 1261–1273 (2015).
Xia, M., Valverde‐Barrantes, O. J., Suseela, V., Blackwood, C. B. & Tharayil, N. Coordination between compound‐specific chemistry and morphology in plant roots aligns with ancestral mycorrhizal association in woody angiosperms. N. Phytol. 232, 1259–1271 (2021).
Thamer, S., Schädler, M., Bonte, D. & Ballhorn, D. J. Dual benefit from a belowground symbiosis: nitrogen fixing rhizobia promote growth and defense against a specialist herbivore in a cyanogenic plant. Plant Soil 341, 209–219 (2011).
Bai, Y. & Cotrufo, M. F. Grassland soil carbon sequestration: current understanding, challenges, and solutions. Science 377, 603–608 (2022).
Ha, K. V., Marschner, P. & Bünemann, E. K. Dynamics of C, N, P and microbial community composition in particulate soil organic matter during residue decomposition. Plant Soil 303, 253–264 (2008).
Jo, I., Fei, S., Oswalt, C. M., Domke, G. M. & Phillips, R. P. Shifts in dominant tree mycorrhizal associations in response to anthropogenic impacts. Sci. Adv. 5, eaav6358 (2019).
Pregitzer, K. S. et al. Fine root architecture of nine North American trees. Ecol. Monogr. 72, 293–309 (2002).
Yan, H. et al. Mycorrhizal symbiosis pathway and edaphic fertility frame root economics space among tree species. N. Phytol. 234, 1639–1653 (2022).
Carmona, C. P. et al. Fine-root traits in the global spectrum of plant form and function. Nature 597, 683–687 (2021).
Guerrero‐Ramírez, N. R. et al. Global Root Traits (GRooT) database. Glob. Ecol. Biogeogr. 30, 25–37 (2021).
Soudzilovskaia, N. A. et al. FungalRoot v.2.0 – an empirical database of plant mycorrhizal traits: a response to Bueno et al. (2021) ‘Towards a consistent benchmark for plant mycorrhizal association databases’. N. Phytol. 235, 1689–1691 (2022).
Shangguan, W., Dai, Y., Duan, Q., Liu, B. & Yuan, H. A global soil data set for earth system modeling. J. Adv. Model. Earth Syst. 6, 249–263 (2014).
The global soil dataset for Earth system modeling. Beijing Normal University http://globalchange.bnu.edu.cn/research/soilw#download (2014).
Dinerstein, E. et al. An ecoregion-based approach to protecting half the terrestrial realm. BioScience 67, 534–545 (2017).
Jackson, R. B. et al. A global analysis of root distributions for terrestrial biomes. Oecologia 108, 389–411 (1996).
McCormack, M. L., Adams, T. S., Smithwick, E. A. H. & Eissenstat, D. M. Variability in root production, phenology, and turnover rate among 12 temperate tree species. Ecology 95, 2224–2235 (2014).
Hansson, K., Helmisaari, H.-S., Sah, S. P. & Lange, H. Fine root production and turnover of tree and understorey vegetation in Scots pine, silver birch and Norway spruce stands in SW Sweden. For. Ecol. Manag. 309, 58–65 (2013).
McCormack, M. L., Eissenstat, D. M., Prasad, A. M. & Smithwick, E. A. H. Regional scale patterns of fine root lifespan and turnover under current and future climate. Glob. Change Biol. 19, 1697–1708 (2013).
MODIS/Terra+Aqua land cover type yearly L3 global 500m sin grid V061. US Geological Survey https://lpdaac.usgs.gov/products/mcd12q1v061/ (2022).
Lehmann, J. & Kleber, M. The contentious nature of soil organic matter. Nature 528, 60–68 (2015).
Wu, Q. et al. Substrate and climate determine terrestrial litter decomposition. Proc. Natl Acad. Sci. USA 122, e2420664122 (2025).
Zhang, H., Yuan, W., Dong, W. & Liu, S. Seasonal patterns of litterfall in forest ecosystem worldwide. Ecol. Complex. 20, 240–247 (2014).
Li, S. et al. Benchmark estimates for aboveground litterfall data derived from ecosystem models. Environ. Res. Lett. 14, 084020 (2019).
Global average aboveground litterfall data from 1982 to 2013. National Ecosystem Science Data Center http://www.nesdc.org.cn/sdo/detail?id=618a22467e2817667e3c7d9e (2019).
Lai, J., Zou, Y., Zhang, J. & Peres‐Neto, P. R. Generalizing hierarchical and variation partitioning in multiple regression and canonical analyses using the rdacca.hp R package. Methods Ecol. Evol. 13, 782–788 (2022).
Bates, D., Mächler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48 (2015).
Ma, N. Dynamic processes and the iterative effect of the finest roots on forest soil carbon accrual in the Northern Hemisphere. figshare https://doi.org/10.6084/m9.figshare.26995555.v3 (2025).
Acknowledgements
The authors thank H. Z. Ma, L. J. Xiao and X.L. Fan for providing the reference data of belowground plant biomass, belowground net primary productivity and microbial decomposition rate, P. X. Wang, C. Z. Hu, L. Y. Zhang and L. Jiang for root data collection and J. L. Peng for providing R code assistance. L.K. and S.N. acknowledge funding support from the National Key Research and Development Program of China (grant no. 2022YFF0802100); L.K. from the National Natural Science Foundation of China (grant no. 32222059), H.W. from the National Natural Science Foundation of China (grant no. 32330071), G.T.F. from Laboratoires d’Excellence (LabEx) TULIP (grant no. ANR-10-LABX-41) and P.B.R. from the US National Science Foundation ASCEND Biology Integration Institute (grant no. NSF-DBI-2021898).
Author information
Authors and Affiliations
Contributions
L.K., S.L. and N.M. conceptualized the work. N.M., S.L., L.K., M.Z., R.Z., M.L.M., G.T.F., D.G. and B.Z. performed the data analyses. L.K., S.L., N.M., M.L.M., G.T.F., P.C., H.W., S.N., P.B.R., M.Z., R.Z., B.Z., D.G., A.G., Y.H., J.G., X.F., X.D., S.M., J.Z. and F.Y. contributed to framing and interpretation. N.M., L.K., M.Z. and R.Z. performed the data visualization. N.M., L.K. and S.L. wrote the original draft. All authors reviewed and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Geoscience thanks Björn Berg and Cindy Prescott for their contribution to the peer review of this work. Primary Handling Editors: Tamara Goldin and Xujia Jiang, in collaboration with the Nature Geoscience team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
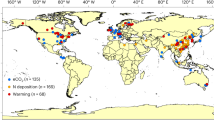
Extended Data Fig. 1 Geographic locations of 328 forest sites across the Northern Hemisphere.
Different colors show three absorptive root processes. The size of each point indicates the number of observations in the same forest site.
Extended Data Fig. 2 Absorptive root-derived C flow and first-year absorptive root mass loss.
(a) Carbon inflow (0.66 PgC, green bar) and outflow (0.14 PgC, the light green part) are derived from annual absorptive root dynamics. (b) The percentage of absorptive root litter mass loss in first-year decomposition. Dots represent values of individual observations, and n represents the number of observations. The black point represents the mean value. The vertical black line represents the standard deviation around the mean value.
Extended Data Fig. 3 Microbial-derived soil C accrual through their iterative dynamics on a yearly scale.
Grey bars show soil C accrual of absorptive root dynamics through iterative effects across years.
Extended Data Fig. 4 Individual effects of seven predictors on absorptive root (a) biomass, (b) turnover, and (c) decomposition quantified by the hierarchical and variation partitioning for canonical analysis.
Two-sided permutation tests (n = 999) were used to assess statistical significance, without multiple comparisons. MAT, mean annual temperature; MAP, mean annual precipitation; TN, soil total nitrogen; TP, soil total phosphorus. Mycorrhizal types refer to arbuscular mycorrhizal or ectomycorrhizal; phylogeny refers to gymnosperm or angiosperm; leaf habit types refer to evergreen or deciduous species.
Extended Data Fig. 5 Trait-process relationships in absorptive roots.
All variables are log10-transformed. Dots represent values of species-mean levels and error bars show standard error around species-mean values. The grey shading around each regression line represents the 95% confidence interval. (a), (d), and (g) show relationships of root diameter (RD, mm) with root biomass (g m−2), turnover (yr−1), and decomposition rate (yr−1) by fitting linear regressions. (b), (e), and (h) show relationships of root tissue density (RTD, g cm−3) with root biomass (g m−2), turnover (yr−1), and decomposition rate (yr−1) by fitting linear regressions. (c), (f), and (i) show relationships of root nitrogen (N) concentration (mg g−1) with root biomass (g m−2), turnover (yr−1), and decomposition rate (yr−1) by fitting linear regressions. Statistical significance (P < 0.05) was tested with two-sided t-tests without multiple-comparison correction.
Extended Data Fig. 6 Relationships of traits with lifespan- and production-based turnover in absorptive roots.
The absorptive root traits include root diameter (mm), specific root length (SRL, m g−1), root tissue density (RTD, g cm−3), and root nitrogen (N, mg g−1) concentration. All variables are log10-transformed, except variables in (d). Dots represent values of species-mean levels and error bars show standard error around species-mean values. The grey shading around each regression line represents the 95% confidence interval. (a), (c), (e), and (g) show relationships between root traits and lifespan-based root turnover (yr−1) by fitting linear regressions. (b), (d), (f), and (h) show relationships between root traits and production-based root turnover (yr−1) by fitting linear regressions. Statistical significance (P < 0.05) was tested with two-sided t-tests without multiple-comparison correction.
Supplementary information
Supplementary Information
Supplementary Methods, Tables 1–5, Fig. 1 and Discussion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ma, N., Li, S., McCormack, M.L. et al. Substantial forest soil carbon accrual from absorptive fine roots over decadal timescales. Nat. Geosci. 18, 1020–1026 (2025). https://doi.org/10.1038/s41561-025-01790-5
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41561-025-01790-5