Abstract
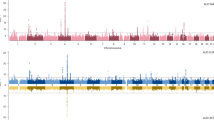
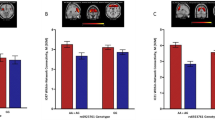
Despite neurobiological overlap, alcohol use disorder (AUD) and body mass index (BMI) show minimal genetic correlation (rg), possibly due to mixed directions of shared variants. Here we applied MiXeR to investigate shared genetic architecture between AUD and BMI, conjunctional false discovery rate to detect shared loci and their directional effect, local analysis of (co)variant association for local rg, functional mapping and annotation to identify lead single-nucleotide polymorphisms, Genotype-Tissue Expression (GTEx) to examine tissue enrichment and BrainXcan to assess associations with brain phenotypes. MiXeR indicated 82.2% polygenic overlap, despite an rg of −0.03. The conjuctional false discovery rate method identified 132 shared lead single-nucleotide polymorphisms, with 53 novel, showing both concordant and discordant effects. GTEx analyses identified overexpression in multiple brain regions. Amygdala and caudate nucleus volumes were associated with AUD and BMI. Opposing variant effects explain the minimal rg between AUD and BMI, with implicated brain regions involved in executive function and reward, clarifying their polygenic overlap and neurobiological mechanisms.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Full summary statistics from two genome-wide association studies are available at the following locations: GIANT Consortium website (https://portals.broadinstitute.org/collaboration/giant/index.php/GIANT_consortium_data_files) and the Gelernter Lab website without restriction (https://medicine.yale.edu/lab/gelernter/stats/) or dbGaP (accession number phs001672, under the ‘Addiction’ analysis; registration and approval are needed following dbGaP’s data accessing process). Researchers seeking access to the exact AUD summary statistics cohort used in this study should contact the original study authors for more information (Zhou et al., 2023 (ref. 23)). Replication summary statistics are available via the FinnGen website (https://www.finngen.fi/en/access_results). Annotations for the lead SNPs corresponding to VEP, CADD scores and nearest transcription start site were sourced from OpenTargets (https://genetics.opentargets.org/, v22.10). The presence of lead SNPs within genes was confirmed using dbGaP (https://www.ncbi.nlm.nih.gov/gap/). The TCRD and OpenTargets were accessed to integrate drug–protein interaction/druggability information (https://pharos.nih.gov/, v3.18.0; https://platform.opentargets.org/, v23.12).
References
Apovian, C. M. Obesity: definition, comorbidities, causes, and burden. Am. J. Manag. Care 22, s176–s185 (2016).
Sacks, J. J., Gonzales, K. R., Bouchery, E. E., Tomedi, L. E. & Brewer, R. D. 2010 national and state costs of excessive alcohol consumption. Am. J. Prev. Med. 49, e73–e79 (2015).
Kranzler, H. R. Overview of alcohol use disorder. Am. J. Psychiatry 180, 565–572 (2023).
Hruby, A. et al. Determinants and consequences of obesity. Am. J. Public Health 106, 1656–1662 (2016).
Raza, S. A., Sokale, I. O. & Thrift, A. P. Burden of high-risk phenotype of heavy alcohol consumption among obese U.S. population: results from National Health and Nutrition Examination Survey, 1999–2020. Lancet Reg. Health Am. 23, 100525 (2023).
Magkos, F. et al. On the pathogenesis of obesity: causal models and missing pieces of the puzzle. Nat. Metab. 6, 1856–1865 (2024).
Yohn, S. E., Galbraith, J., Calipari, E. S. & Conn, P. J. Shared behavioral and neurocircuitry disruptions in drug addiction, obesity, and binge eating disorder: focus on group I mGluRs in the mesolimbic dopamine pathway. ACS Chem. Neurosci. 10, 2125–2143 (2019).
Volkow, N. D. et al. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. NeuroImage 42, 1537–1543 (2008).
Volkow, N. D., Wise, R. A. & Baler, R. The dopamine motive system: implications for drug and food addiction. Nat. Rev. Neurosci. 18, 741–752 (2017).
Moore, C. F., Sabino, V., Koob, G. F. & Cottone, P. Pathological overeating: emerging evidence for a compulsivity construct. Neuropsychopharmacology 42, 1375–1389 (2017).
Lindgren, E. et al. Food addiction: a common neurobiological mechanism with drug abuse. FBL 23, 811–836 (2018).
Gearhardt, A. N. & Hebebrand, J. The concept of “food addiction” helps inform the understanding of overeating and obesity: Debate Consensus. Am. J. Clin. Nutr. 113, 274–276 (2021).
Deschaine, S. L. & Leggio, L. From “hunger hormone” to “it’s complicated”: ghrelin beyond feeding control. Physiology 37, 5–15 (2022).
Farokhnia, M., Faulkner, M. L., Piacentino, D., Lee, M. R. & Leggio, L. Ghrelin: from a gut hormone to a potential therapeutic target for alcohol use disorder. Physiol. Behav. 204, 49–57 (2019).
Klausen, M. K., Thomsen, M., Wortwein, G. & Fink-Jensen, A. The role of glucagon-like peptide 1 (GLP-1) in addictive disorders. Br. J. Pharmacol. 179, 625–641 (2022).
Leggio, L. et al. GLP-1 receptor agonists are promising but unproven treatments for alcohol and substance use disorders. Nat. Med. 29, 2993–2995 (2023).
Elmaleh-Sachs, A. et al. Obesity management in adults: a review. JAMA 330, 2000–2015 (2023).
Perry, C. M. P. et al. The management of substance use disorders: synopsis of the 2021 U.S. Department of Veterans Affairs and U.S. Department of Defense Clinical Practice Guideline. Ann. Intern. Med. 175, 720–731 (2022).
Reus, V. I. et al. The American Psychiatric Association Practice Guideline for the pharmacological treatment of patients with alcohol use disorder. Am. J. Psychiatry 175, 86–90 (2018).
Verhulst, B., Neale, M. C. & Kendler, K. S. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychol. Med. 45, 1061–1072 (2015).
Loos, R. J. F. & Yeo, G. S. H. The genetics of obesity: from discovery to biology. Nat. Rev. Genet. 23, 120–133 (2022).
Saunders, G. R. B. et al. Genetic diversity fuels gene discovery for tobacco and alcohol use. Nature 612, 720–724 (2022).
Zhou, H. et al. Multi-ancestry study of the genetics of problematic alcohol use in over 1 million individuals. Nat. Med. 29, 3184–3192 (2023).
Frei, O. et al. Bivariate causal mixture model quantifies polygenic overlap between complex traits beyond genetic correlation. Nat. Commun. 10, 2417 (2019).
Hindley, G. et al. Charting the landscape of genetic overlap between mental disorders and related traits beyond genetic correlation. Am. J. Psychiatry 179, 833–843 (2022).
Tesfaye, M. et al. Shared genetic architecture between irritable bowel syndrome and psychiatric disorders reveals molecular pathways of the gut–brain axis. Genome Med. 15, 60 (2023).
Demontis, D. et al. Genome-wide analyses of ADHD identify 27 risk loci, refine the genetic architecture and implicate several cognitive domains. Nat. Genet. 55, 198–208 (2023).
Hope, S. et al. Bidirectional genetic overlap between autism spectrum disorder and cognitive traits. Transl. Psychiatry 13, 295 (2023).
Werme, J., van der Sluis, S., Posthuma, D. & de Leeuw, C. A. An integrated framework for local genetic correlation analysis. Nat. Genet. 54, 274–282 (2022).
Smeland, O. B. et al. Discovery of shared genomic loci using the conditional false discovery rate approach. Hum. Genet. 139, 85–94 (2020).
Chang, R. et al. Emerging roles of FTO in neuropsychiatric disorders. BioMed Res. Int. 2022, 2677312 (2022).
Pickrell, J. K. et al. Detection and interpretation of shared genetic influences on 42 human traits. Nat. Genet. 48, 709–717 (2016).
Pasman, J. A. et al. The CADM2 gene and behavior: a phenome-wide scan in UK-Biobank. Behav. Genet. 52, 306–314 (2022).
Sanchez-Roige, S. et al. CADM2 is implicated in impulsive personality and numerous other traits by genome- and phenome-wide association studies in humans and mice. Transl. Psychiatry 13, 167 (2023).
Bahrami, S. et al. Dissecting the shared genetic basis of migraine and mental disorders using novel statistical tools. Brain 145, 142–153 (2022).
Rødevand, L. et al. Characterizing the shared genetic underpinnings of schizophrenia and cardiovascular disease risk factors. Am. J. Psychiatry 180, 815–826 (2023).
Wiström, E. D. et al. Genome-wide analysis reveals genetic overlap between alcohol use behaviours, schizophrenia and bipolar disorder and identifies novel shared risk loci. Addiction 117, 600–610 (2022).
Kircher, M. et al. A general framework for estimating the relative pathogenicity of human genetic variants. Nat. Genet. 46, 310–315 (2014).
Aguet, F. et al. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science 369, 1318–1330 (2020).
La Manno, G. et al. Molecular diversity of midbrain development in mouse, human, and stem cells. Cell 167, 566–580 (2016).
Kelleher, K. J. et al. Pharos 2023: an integrated resource for the understudied human proteome. Nucleic Acids Res. 51, D1405–D1416 (2023).
Warren, S., Capra, N. F. & Yezierski, R. P. in Fundamental Neuroscience for Basic and Clinical Applications 5th edn (eds Haines, D. E. & Mihailoff, G. A.) Ch. 17, 243–257 (Elsevier, 2018).
Bahrami, S. et al. Shared genetic loci between body mass index and major psychiatric disorders: a genome-wide association study. JAMA Psychiatry 77, 503–512 (2020).
Torgersen, K. A.-O. et al. Shared genetic loci between depression and cardiometabolic traits. PLoS Genet. 18, e1010161 (2022).
Kranzler, H. R. et al. Genome-wide association study of alcohol consumption and use disorder in 274,424 individuals from multiple populations. Nat. Commun. 10, 1499 (2019).
Loos, R. J. F. & Yeo, G. S. H. The bigger picture of FTO—the first GWAS-identified obesity gene. Nat. Rev. Endocrinol. 10, 51–61 (2014).
Laber, S. et al. Linking the FTO obesity rs1421085 variant circuitry to cellular, metabolic, and organismal phenotypes in vivo. Sci. Adv. 7, eabg0108 (2021).
Zhang, Z. et al. The rs1421085 variant within FTO promotes brown fat thermogenesis. Nat. Metab. 5, 1337–1351 (2023).
Hess, M. E. et al. The fat mass and obesity associated gene (Fto) regulates activity of the dopaminergic midbrain circuitry. Nat. Neurosci. 16, 1042–1048 (2013).
Zhang, X. et al. Structural insights into FTO’s catalytic mechanism for the demethylation of multiple RNA substrates. Proc. Natl Acad. Sci. USA 116, 2919–2924 (2019).
Nebert, D. W. & Liu, Z. SLC39A8 gene encoding a metal ion transporter: discovery and bench to bedside. Hum. Genomics 13, 51 (2019).
Sazonovs, A. et al. Large-scale sequencing identifies multiple genes and rare variants associated with Crohn’s disease susceptibility. Nat. Genet. 54, 1275–1283 (2022).
Sunuwar, L. et al. Pleiotropic ZIP8 A391T implicates abnormal manganese homeostasis in complex human disease. JCI Insight 5, e140978 (2020).
Arends, R. M. et al. Associations between the CADM2 gene, substance use, risky sexual behavior, and self-control: a phenome-wide association study. Addict. Biol. 26, e13015 (2021).
Koller, D. et al. Pleiotropy and genetically inferred causality linking multisite chronic pain to substance use disorders. Mol. Psychiatry 29, 2021–2030 (2024).
Morris, J. et al. Genetic variation in CADM2 as a link between psychological traits and obesity. Sci. Rep. 9, 7339 (2019).
Grodin, E. N. et al. Ibudilast, a neuroimmune modulator, reduces heavy drinking and alcohol cue-elicited neural activation: a randomized trial. Transl. Psychiatry 11, 355 (2021).
Grigsby, K. B. et al. Preclinical and clinical evidence for suppression of alcohol intake by apremilast. J. Clin. Invest. 133, e159103 (2023).
Crocetti, L., Floresta, G., Cilibrizzi, A. & Giovannoni, M. P. An overview of PDE4 inhibitors in clinical trials: 2010 to early 2022. Molecules 27, 4964 (2022).
Ferguson, L. D. et al. Effect of the phosphodiesterase 4 inhibitor apremilast on cardiometabolic outcomes in psoriatic disease—results of the Immune Metabolic Associations in Psoriatic Arthritis study. Rheumatology 61, 1026–1034 (2021).
Wu, C. & Rajagopalan, S. Phosphodiesterase-4 inhibition as a therapeutic strategy for metabolic disorders. Obes. Rev. 17, 429–441 (2016).
Grunvald, E. et al. AGA clinical practice guideline on pharmacological interventions for adults with obesity. Gastroenterology 163, 1198–1225 (2022).
Vengeliene, V. & Spanagel, R. mGlu2 mechanism-based interventions to treat alcohol relapse. Front. Pharmacol. 13, 985954 (2022).
Augier, E. et al. The mGluR2 positive allosteric modulator, AZD8529, and cue-induced relapse to alcohol seeking in rats. Neuropsychopharmacology 41, 2932–2940 (2016).
Sidhpura, N., Weiss, F. & Martin-Fardon, R. Effects of the mGlu2/3 agonist LY379268 and the mGlu5 antagonist MTEP on ethanol seeking and reinforcement are differentially altered in rats with a history of ethanol dependence. Biol. Psychiatry 67, 804–811 (2010).
Johnson, K. A. & Lovinger, D. M. Allosteric modulation of metabotropic glutamate receptors in alcohol use disorder: insights from preclinical investigations. Adv. Pharmacol. 88, 193–232 (2020).
Windisch, K. A. & Czachowski, C. L. Effects of group II metabotropic glutamate receptor modulation on ethanol- and sucrose-seeking and consumption in the rat. Alcohol 66, 77–85 (2018).
Gómez-Apo, E., Mondragón-Maya, A., Ferrari-Díaz, M. & Silva-Pereyra, J. Structural brain changes associated with overweight and obesity. J. Obes. 2021, 6613385 (2021).
Meng, X., Huang, D., Ao, H., Wang, X. & Gao, X. Food cue recruits increased reward processing and decreased inhibitory control processing in the obese/overweight: an activation likelihood estimation meta-analysis of fMRI studies. Obes. Res. Clin. Pract. 14, 127–135 (2020).
Donnelly, B. et al. Neuroimaging in bulimia nervosa and binge eating disorder: a systematic review. J. Eat. Disord. 6, 3 (2018).
Giel, K. E. et al. Binge eating disorder. Nat. Rev. Dis. Primers 8, 16 (2022).
Fritz, M., Klawonn, A. M. & Zahr, N. M. Neuroimaging in alcohol use disorder: from mouse to man. J. Neurosci. Res. 100, 1140–1158 (2022).
Koob, G. F. & Volkow, N. D. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3, 760–773 (2016).
Koob, G. F. & Volkow, N. D. Neurocircuitry of addiction. Neuropsychopharmacology 35, 217–238 (2010).
Doi, T., Fan, Y., Gold, J. I. & Ding, L. The caudate nucleus contributes causally to decisions that balance reward and uncertain visual information. eLife 9, e56694 (2020).
Gupta, A., Osadchiy, V. & Mayer, E. A. Brain–gut–microbiome interactions in obesity and food addiction. Nat. Rev. Gastroenterol. Hepatol. 17, 655–672 (2020).
Stover, P. J. et al. Neurobiology of eating behavior, nutrition, and health. J. Intern. Med. 294, 582–604 (2023).
Bodell, L. P. & Racine, S. E. A mechanistic staging model of reward processing alterations in individuals with binge-type eating disorders. Int. J. Eat. Disord. 56, 516–522 (2023).
Morales, I. Brain regulation of hunger and motivation: the case for integrating homeostatic and hedonic concepts and its implications for obesity and addiction. Appetite 177, 106146 (2022).
Morales, I. & Berridge, K. C. ‘Liking’ and ‘wanting’ in eating and food reward: Brain mechanisms and clinical implications. Physiol. Behav. 227, 113152 (2020).
Siletti, K. et al. Transcriptomic diversity of cell types across the adult human brain. Science 382, eadd7046 (2023).
Fryar, C. D., Carroll, M. D. & Afful, J. Prevalence of Overweight, Obesity, and Extreme Obesity among Adults: United States, Trends 1960–1962 through 2009–2010 (National Center for Health Statistics, 2012).
Pulit, S. L. et al. Meta-analysis of genome-wide association studies for body fat distribution in 694 649 individuals of European ancestry. Hum. Mol. Genet. 28, 166–174 (2018).
Munn-Chernoff, M. A. et al. Shared genetic risk between eating disorder- and substance-use-related phenotypes: evidence from genome-wide association studies. Addict. Biol. 26, e12880 (2021).
Polimanti, R. et al. Genome-wide association study of body mass index in subjects with alcohol dependence. Addict. Biol. 22, 535–549 (2017).
Lofton, H., Ard, J. D., Hunt, R. R. & Knight, M. G. Obesity among African American people in the United States: a review. Obesity 31, 306–315 (2023).
Ogden, C. L. et al. Trends in obesity prevalence by race and Hispanic Origin—1999–2000 to 2017–2018. JAMA 324, 1208–1210 (2020).
Zapolski, T. C. B., Pedersen, S. L., McCarthy, D. M. & Smith, G. T. Less drinking, yet more problems: understanding African American drinking and related problems. Psychol. Bull. 140, 188–223 (2014).
Agrawal, A. et al. The collaborative study on the genetics of alcoholism: overview. Genes Brain Behav. 22, e12864 (2023).
Yengo, L. et al. Meta-analysis of genome-wide association studies for height and body mass index in ∼700000 individuals of European ancestry. Hum. Mol. Genet. 27, 3641–3649 (2018).
Zhou, H. et al. Genome-wide meta-analysis of problematic alcohol use in 435,563 individuals yields insights into biology and relationships with other traits. Nat. Neurosci. 23, 809–818 (2020).
Holland, D. et al. Beyond SNP heritability: polygenicity and discoverability of phenotypes estimated with a univariate Gaussian mixture model. PLoS Genet. 16, e1008612 (2020).
Howard, D. M. et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat. Neurosci. 22, 343–352 (2019).
Trubetskoy, V. et al. Mapping genomic loci implicates genes and synaptic biology in schizophrenia. Nature 604, 502–508 (2022).
Cuellar-Partida, G. et al. Genome-wide association study identifies 48 common genetic variants associated with handedness. Nat. Hum. Behav. 5, 59–70 (2021).
Bulik-Sullivan, B. K. et al. LD score regression distinguishes confounding from polygenicity in genome-wide association studies. Nat. Genet. 47, 291–295 (2015).
Andreassen, O. A. et al. Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. Am. J. Hum. Genet. 92, 197–209 (2013).
Kurki, M. I. et al. FinnGen provides genetic insights from a well-phenotyped isolated population. Nature 613, 508–518 (2023).
Cheng, W. et al. Shared genetic architecture between schizophrenia and subcortical brain volumes implicates early neurodevelopmental processes and brain development in childhood. Mol. Psychiatry 27, 5167–5176 (2022).
Watanabe, K., Taskesen, E., van Bochoven, A. & Posthuma, D. Functional mapping and annotation of genetic associations with FUMA. Nat. Commun. 8, 1826 (2017).
Auton, A. et al. A global reference for human genetic variation. Nature 526, 68–74 (2015).
Ochoa, D. et al. The next-generation Open Targets Platform: reimagined, redesigned, rebuilt. Nucleic Acids Res. 51, D1353–D1359 (2023).
Liang, Y. et al. BrainXcan identifies brain features associated with behavioral and psychiatric traits using large scale genetic and imaging data. Preprint at medRxiv https://doi.org/10.1101/2021.06.01.21258159 (2021).
Acknowledgements
We thank all of the research participants who contributed their data to the data sources used in this study. This work was supported by funding from the National Institute on Alcohol Abuse and Alcoholism (R01 AA030041 to J.C.G., H.R.K. and C.T.R.; and R01 AA030056 to H.R.K.), the Department of Defense (HU0001-22-2-0066 to J.C.G., H.R.K. and C.T.R.) and the Veterans Integrated Service Network 4 Mental Illness Research, Education and Clinical Center of the Crescenz Veterans Affairs Medical Center (to H.R.K. and A.J.). L.L. is a federal employee and is supported by the National Institute on Drug Abuse and the National Institute on Alcohol Abuse and Alcoholism Intramural Research Programs. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the paper. The opinions and assertions herein are those of the authors and do not necessarily reflect the official views of the Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. Moreover, the opinions and assertions herein do not necessarily reflect the official views of the Department of Defense, Uniformed Services University, the National Institute on Alcohol Abuse and Alcoholism or the US Government and do not imply endorsement by the Federal Government.
Author information
Authors and Affiliations
Contributions
Study concept and design: J.C.G. Analysis of data: Z.P., C.N.D., J.C.G. and H.Z. Drafting of the paper: S.G.M., J.C.G., M.R.S., C.N.D., S.T., H.R.K. and E.L.W. Critical revision of the paper for important intellectual content: C.N.D., S.G.M., J.C.G., H.R.K., C.T.R., L.L., S.T., H.Z., A.J. and J.G. Final approval of the paper: all.
Corresponding author
Ethics declarations
Competing interests
H.R.K. is a member of advisory boards for Altimmune, Clearmind Medicine, Dicerna Pharmaceuticals, Enthion Pharmaceuticals, Lilly Pharmaceuticals and Sophrosyne Pharmaceuticals; a consultant to Sobrera Pharmaceuticals and Altimmune; the recipient of research funding and medication supplies for an investigator-initiated study from Alkermes; and a member of the American Society of Clinical Psychopharmacology’s Alcohol Clinical Trials Initiative, which was supported in the past 3 years by Alkermes, Dicerna, Ethypharm, Imbrium, Indivior, Kinnov, Lilly, Otsuka and Pear. J.G. and H.R.K. hold US patent 10,900,082 titled ‘Genotype-guided dosing of opioid agonists’, issued 26 January 2021. J.G. is paid for editorial work for the journal Complex Psychiatry. The other authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Shirley Hill and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Malone, S.G., Davis, C.N., Piserchia, Z. et al. Alcohol use disorder and body mass index show genetic pleiotropy and shared neural associations. Nat Hum Behav 9, 1056–1066 (2025). https://doi.org/10.1038/s41562-025-02148-y
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41562-025-02148-y
This article is cited by
-
Crosstalk between alcohol use disorder and obesity: two sides of the same coin?
Molecular Psychiatry (2025)