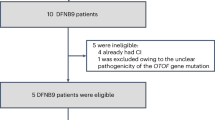
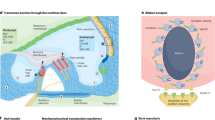
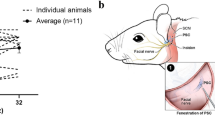
Abstract
Individuals with congenital deafness that have received gene therapy represent a unique group who experience hearing recovery and speech development. However, it is unclear how hearing-related cortex changes because of gene therapy. Here we study neural processing in ten patients using functional near-infrared spectroscopy and electroencephalography during a six-month follow-up period. Patients showed an enhancement of activation in the auditory cortex, particularly in parts of the Sylvian parietotemporal area while listening to music. Activation in the right anterior temporal lobe and left Sylvian parietotemporal area was also enhanced when listening to speech. The electroencephalography data showed that the power of the resting-state electroencephalography beta band at time points T2 and T3 was statistically significantly increased after gene therapy, and mismatch negativity amplitudes at T2 and T3 were statistically significantly higher than those at T0. The mental developmental level of the patients also increased after gene therapy. These preliminary findings illuminate the neural and cognitive effects of gene therapy, supporting its potential effectiveness in auditory and mental development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
To respect the privacy of the participants, individual patient data are anonymized. The de-identified data (text, figures, tables and appendices) are available in the manuscript. The detailed data analysis is available in the Supplementary Information. Requests for more information on the trial should be directed to the corresponding author.
Code availability
Requests for the code used should be directed to the corresponding author.
References
Lieu, J. E. C., Kenna, M., Anne, S. & Davidson, L. Hearing loss in children: a review. JAMA 324, 2195–2205 (2020).
Morton, C. C. & Nance, W. E. Newborn hearing screening—a silent revolution. N. Engl. J. Med. 354, 2151–2164 (2006).
Chen, L. C., Stropahl, M., Schonwiesner, M. & Debener, S. Enhanced visual adaptation in cochlear implant users revealed by concurrent EEG–fNIRS. NeuroImage 146, 600–608 (2017).
Anderson, C. A., Cushing, S. L., Papsin, B. C. & Gordon, K. A. Cortical imbalance following delayed restoration of bilateral hearing in deaf adolescents. Hum. Brain Mapp. 43, 3662–3679 (2022).
Sevy, A. B. et al. Neuroimaging with near-infrared spectroscopy demonstrates speech-evoked activity in the auditory cortex of deaf children following cochlear implantation. Hear. Res. 270, 39–47 (2010).
Anderson, C. A., Wiggins, I. M., Kitterick, P. T. & Hartley, D. E. H. Adaptive benefit of cross-modal plasticity following cochlear implantation in deaf adults. Proc. Natl Acad. Sci. USA 114, 10256–10261 (2017).
Lee, H. J. et al. Preoperative differences of cerebral metabolism relate to the outcome of cochlear implants in congenitally deaf children. Hear. Res. 203, 2–9 (2005).
Zeng, F. G., Rebscher, S., Harrison, W., Sun, X. & Feng, H. Cochlear implants: system design, integration, and evaluation. IEEE Rev. Biomed. Eng. 1, 115–142 (2008).
Lv, J. et al. AAV1-hOTOF gene therapy for autosomal recessive deafness 9: a single-arm trial. Lancet 403, 2317–2325 (2024).
Wang, H. et al. Bilateral gene therapy in children with autosomal recessive deafness 9: single-arm trial results. Nat. Med. 30, 1898–1904 (2024).
Qi, J. et al. AAV-mediated gene therapy restores hearing in patients with DFNB9 deafness. Adv. Sci. (Weinh.) 11, e2306788 (2024).
Zeng, F.-G. Gene therapy restored partial hearing in a dozen deaf children. Hear. J. 77, 1,2 (2024).
Strenzke, N. A cure for deafness? Med 5, 285–287 (2024).
Dewey, R. S. & Hartley, D. E. Cortical cross-modal plasticity following deafness measured using functional near-infrared spectroscopy. Hear. Res. 325, 55–63 (2015).
Sandmann, P. et al. Rapid bilateral improvement in auditory cortex activity in postlingually deafened adults following cochlear implantation. Clin. Neurophysiol. 126, 594–607 (2015).
Sharma, A., Campbell, J. & Cardon, G. Developmental and cross-modal plasticity in deafness: evidence from the P1 and N1 event related potentials in cochlear implanted children. Int. J. Psychophysiol. 95, 135–144 (2015).
Quaresima, V., Bisconti, S. & Ferrari, M. A brief review on the use of functional near-infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain Lang. 121, 79–89 (2012).
Gagnon, L. et al. Quantification of the cortical contribution to the NIRS signal over the motor cortex using concurrent NIRS–fMRI measurements. NeuroImage 59, 3933–3940 (2012).
Pollonini, L. et al. Auditory cortex activation to natural speech and simulated cochlear implant speech measured with functional near-infrared spectroscopy. Hear. Res. 309, 84–93 (2014).
Anderson, C. A., Wiggins, I. M., Kitterick, P. T. & Hartley, D. E. H. Pre-operative brain imaging using functional near-infrared spectroscopy helps predict cochlear implant outcome in deaf adults. J. Assoc. Res. Otolaryngol. 20, 511–528 (2019).
Wang, Y. et al. Differential auditory cortical development in left and right cochlear implanted children. Cereb. Cortex 32, 5438–5454 (2022).
Zhou, X. et al. Cortical speech processing in postlingually deaf adult cochlear implant users, as revealed by functional near-infrared spectroscopy. Trends Hear. 22, 2331216518786850 (2018).
Zhou, X. Q. et al. Cortical responses correlate with speech performance in pre-lingually deaf cochlear implant children. Front. Neurosci. 17, 1126813 (2023).
Lawrence, R. J., Wiggins, I. M., Anderson, C. A., Davies-Thompson, J. & Hartley, D. E. H. Cortical correlates of speech intelligibility measured using functional near-infrared spectroscopy (fNIRS). Hear. Res. 370, 53–64 (2018).
Armony, J. L., Aubé, W., Angulo-Perkins, A., Peretz, I. & Concha, L. The specificity of neural responses to music and their relation to voice processing: an fMRI-adaptation study. Neurosci. Lett. 593, 35–39 (2015).
Whitehead, J. C. & Armony, J. L. Singing in the brain: neural representation of music and voice as revealed by fMRI. Hum. Brain Mapp. 39, 4913–4924 (2018).
Peretz, I., Vuvan, D., Lagrois, M. & Armony, J. L. Neural overlap in processing music and speech. Phil. Trans. R. Soc. B 370, 20140090 (2015).
Hamilton, L. S., Oganian, Y., Hall, J. & Chang, E. F. Parallel and distributed encoding of speech across human auditory cortex. Cell 184, 4626–4639.e4613 (2021).
Hickok, G. & Poeppel, D. The cortical organization of speech processing. Nat. Rev. Neurosci. 8, 393–402 (2007).
Ponton, C. W., Moore, J. K. & Eggermont, J. J. Prolonged deafness limits auditory system developmental plasticity: evidence from an evoked potentials study in children with cochlear implants. Scand. Audiol. Suppl. 51, 13–22 (1999).
Olds, C. et al. Cortical activation patterns correlate with speech understanding after cochlear implantation. Ear Hear. 37, e160–e172 (2016).
Mushtaq, F., Wiggins, I. M., Kitterick, P. T., Anderson, C. A. & Hartley, D. E. H. Investigating cortical responses to noise-vocoded speech in children with normal hearing using functional near-infrared spectroscopy (fNIRS). J. Assoc. Res. Otolaryngol. 22, 703–717 (2021).
Lawrence, R. J., Wiggins, I. M., Hodgson, J. C. & Hartley, D. E. H. Evaluating cortical responses to speech in children: a functional near-infrared spectroscopy (fNIRS) study. Hear. Res. 401, 108155 (2021).
Wang, S. et al. Features of beta-gamma phase-amplitude coupling in cochlear implant users derived from EEG. Hear. Res. 428, 108668 (2023).
Gola, M., Magnuski, M., Szumska, I. & Wróbel, A. EEG beta band activity is related to attention and attentional deficits in the visual performance of elderly patients. Int. J. Psychophysiol. 89, 334–341 (2013).
Turgeon, C., Lazzouni, L., Lepore, F. & Ellemberg, D. An objective auditory measure to assess speech recognition in adult cochlear implant users. Clin. Neurophysiol. 125, 827–835 (2014).
Zhang, F. et al. Mismatch negativity and adaptation measures of the late auditory evoked potential in cochlear implant users. Hear. Res. 275, 17–29 (2011).
Jeong, S. W., Chung, S. H. & Kim, L. S. P1 cortical auditory evoked potential in children with unilateral or bilateral cochlear implants: implication for the timing of second cochlear implantation. Eur. Arch. Otorhinolaryngol. 275, 1759–1765 (2018).
Okada, K. et al. Hierarchical organization of human auditory cortex: evidence from acoustic invariance in the response to intelligible speech. Cereb. Cortex 20, 2486–2495 (2010).
Callan, A. & Callan, D. E. Understanding how the human brain tracks emitted speech sounds to execute fluent speech production. PLoS Biol. 20, e3001533 (2022).
Colletti, L., Mandalà, M., Zoccante, L., Shannon, R. V. & Colletti, V. Infants versus older children fitted with cochlear implants: performance over 10 years. Int. J. Pediatr. Otorhinolaryngol. 75, 504–509 (2011).
Yang, Y. et al. The longitude study on the mental development of congenital hearing-impaired infants and toddlers. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 50, 799–804 (2015).
Dang, J. et al. The value of nonverbal intelligence in cochlear implant. Acta Otolaryngol. 143, 24–27 (2023).
Julien, C. The enigma of Mayer waves: facts and models. Cardiovasc. Res. 70, 12–21 (2006).
Costa, Á. et al. Decoding the attentional demands of gait through EEG gamma band features. PLoS ONE 11, e0154136 (2016).
Griffiths, R. & Huntley, M. Griffiths Mental Development Scales—Revised: Birth to 2 Years (American Psychological Association, 1996).
Griffiths, R. The Abilities of Young Children: A Comprehensive System of Mental Measurement for the First Eight Years of Life (ARICD, 1984).
Wechsler, D. Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV) Vol. 1 (APA PsycTests, 2003).
Acknowledgements
We thank the patients and their families for their participation in and support of the study. This work was supported by the Eye & ENT Hospital of Fudan University. The study was supported by the National Natural Science Foundation of China (grant 82225014 (Y.S.), 82171148 (Y.S.), 62371217 (F.C.), 82192864 (H.L.), 82071048 (B.C.) and 82201306 (Y.S.)), the National Key R&D Program of China (grant 2020YFA0908201 (Y.S.), 2021YFA1101302 (Y.S.), 2023YFA0915000(04) (H.T. and B.Z.)) and 2023YFC2508402 (S.S.), the Science and Technology Commission of Shanghai Municipality (grant 23J31900100 (Y.S.)), Xuhui District Hospital and Site Cooperation Project (grant 23XHYD-05 (Y.S.)), the Shanghai Municipal Education Commission (grant 2023ZKZD12 (Y.S.)), the Construction Foundation of First Class Universities 219 (grant 01270021922201*035 (Z.G.)), the Basic Scientific Research Foundation (grant 02120022124005 (Z.G.)), the Research Projects of Shanghai Municipal Health Committee (2022XD059 (S.S.)) and the Key Patient Construction Project of Shanghai Municipal Health Commission (grant shslczdzk02903 (X.X.)). The study was also funded by Shanghai Refreshgene Therapeutics Co., Ltd. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Y.S., H.L., X.X. and F.C. were the principal investigators of the study and conceived the trial. X.X., H.L., F.C. and Y.S. contributed to the study design. J.L., H.W. and Q.C. enrolled the patients. J.Z., C.H., Y.-w.L., X.C., M.X., L.Z., Z.W. and X.W. collected the data. J.Z., Z.G., C.H. and X.W. analysed the data and wrote the manuscript. J.Z., Z.G., C.H., C.P., X.W., S.S. and Y.S. accessed and verified the data. D.W., S.H., Y.C., H.T., B.Z., S.Y., X.H., L.C., B.C., Z.-Y.C., S.S., X.X., H.L., F.C. and Y.S. contributed to the revision of the manuscript. Y.C., H.T., B.C., Y.S., H.L. and X.X. obtained the funding. L.G., Y.S., D.W. and H.T. confirmed the genotypes of the patients. All authors vouch for the fidelity of the protocol and the accuracy and completeness of the reported data. All authors reviewed and approved the manuscript before submission. The corresponding author had full access to all data in the study and had final responsibility for the decision to submit for publication.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks Shuo Wang and Chen Chi Wu for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–13, Tables 1–3 and Methods.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, J., Guo, Z., Pan, C. et al. Preliminary evidence for enhanced auditory cortex activation and mental development after gene therapy in children with autosomal recessive deafness 9. Nat Hum Behav 9, 1457–1469 (2025). https://doi.org/10.1038/s41562-025-02184-8
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41562-025-02184-8