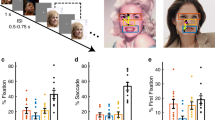
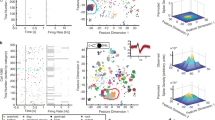
Abstract
Neurons in the human amygdala and hippocampus are classically thought to encode a person’s identity invariant to visual features. However, it remains largely unknown how visual information from higher visual cortical areas is translated into such a semantic representation of an individual person. Here, across four experiments (3,581 neurons from 19 neurosurgical patients over 111 sessions), we demonstrate a region-based feature code for faces, where neurons encode faces on the basis of shared visual features rather than associations of known concepts, contrary to prevailing views. Feature neurons encode groups of faces regardless of their identity, broad semantic categories or familiarity; and the coding regions (that is, receptive fields) predict feature neurons’ response to new face stimuli. Together, our results reveal a new class of neurons that bridge perception-driven representation of facial features with mnemonic semantic representations, which may form the basis for declarative memory.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data supporting the findings of this study, including the CelebA dataset, FBI dataset, FaceGen dataset and monkey dataset, are publicly available on OSF at https://doi.org/10.17605/OSF.IO/36KZC (ref. 63). We also analysed identity neurons from a publicly available human single-neuron dataset (https://doi.org/10.25392/leicester.data.8796335.v1).
Code availability
The source code for this study is publicly available on OSF at https://doi.org/10.17605/OSF.IO/36KZC (ref. 63).
References
Freeman, W. J. Mass Action in The Nervous System (Academic, 1975).
Hinton, G. E., McClelland, J. L. & Rumelhart, D. E. in Parallel Distributed Processing: Explorations in the Microstructure of Cognition Vol. 1 (eds Rumelhart, D. E. & McClelland, J. L.) 77–109 (MIT Press, 1986).
Rolls, E. T., Treves, A. & Tovee, M. J. The representational capacity of the distributed encoding of information provided by populations of neurons in primate temporal visual cortex. Exp. Brain Res. 114, 149–162 (1997).
Churchland, P. S. & Sejnowski, T. J. The Computational Brain (MIT Press, 2016).
Turk, M. A. & Pentland, A. P. Face recognition using eigenfaces. In Proc. 1991 IEEE Computer Society Conference on Computer Vision and Pattern Recognition 586–591 (IEEE, 1991).
Freiwald, W. A., Tsao, D. Y. & Livingstone, M. S. A face feature space in the macaque temporal lobe. Nat. Neurosci. 12, 1187–1196 (2009).
Chang, L. & Tsao, D. Y. The code for facial identity in the primate brain. Cell 169, 1013–1028.e14 (2017).
Bashivan, P., Kar, K. & DiCarlo, J. J. Neural population control via deep image synthesis. Science 364, eaav9436 (2019).
Ponce, C. R. et al. Evolving images for visual neurons using a deep generative network reveals coding principles and neuronal preferences. Cell 177, 999–1009.e10 (2019).
Bao, P. et al. A map of object space in primate inferotemporal cortex. Nature 583, 103–108 (2020).
Loffler, G. et al. fMRI evidence for the neural representation of faces. Nat. Neurosci. 8, 1386–1391 (2005).
Carlin, J. D. & Kriegeskorte, N. Adjudicating between face-coding models with individual-face fMRI responses. PLoS Comput. Biol. 13, e1005604 (2017).
Cao, R. et al. A flexible neural representation of faces in the human brain. Cereb. Cortex Commun. 1, tgaa055 (2020).
Barlow, H. B. Single units and sensation: a neuron doctrine for perceptual psychology? Perception 1, 371–394 (1972).
Valentine, T. A unified account of the effects of distinctiveness, inversion, and race in face recognition. Q. J. Exp. Psychol. A 43, 161–204 (1991).
Quian Quiroga, R. et al. Invariant visual representation by single neurons in the human brain. Nature 435, 1102–1107 (2005).
Quian Quiroga, R. Concept cells: the building blocks of declarative memory functions. Nat. Rev. Neurosci. 13, 587–597 (2012).
De Falco, E. et al. Long-term coding of personal and universal associations underlying the memory web in the human brain. Nat. Commun. 7, 13408 (2016).
Rey, H. G. et al. Encoding of long-term associations through neural unitization in the human medial temporal lobe. Nat. Commun. 9, 4372 (2018).
Bausch, M. et al. Concept neurons in the human medial temporal lobe flexibly represent abstract relations between concepts. Nat. Commun. 12, 6164 (2021).
Rey, H. G. et al. Single neuron coding of identity in the human hippocampal formation. Curr. Biol. 30, 1152–1159.e3 (2020).
Tyree, T. J., Metke, M. & Miller, C. T. Cross-modal representation of identity in the primate hippocampus. Science 382, 417–423 (2023).
Reber, T. P. et al. Representation of abstract semantic knowledge in populations of human single neurons in the medial temporal lobe. PLoS Biol. 17, e3000290 (2019).
Lin, C., Keles, U. & Adolphs, R. Four dimensions characterize attributions from faces using a representative set of English trait words. Nat. Commun. 12, 5168 (2021).
Leopold, D. A., Bondar, I. V. & Giese, M. A. Norm-based face encoding by single neurons in the monkey inferotemporal cortex. Nature 442, 572–575 (2006).
Oosterhof, N. N. & Todorov, A. The functional basis of face evaluation. Proc. Natl Acad. Sci. USA 105, 11087–11092 (2008).
Rutishauser, U. Testing models of human declarative memory at the single-neuron level. Trends Cogn. Sci. 23, 510–524 (2019).
Cao, R. et al. A neuronal code for object representation and memory in the human amygdala and hippocampus. Nat. Commun. 16, 1510 (2025).
Cao, R. et al. Neural mechanisms of face familiarity and learning in the human amygdala and hippocampus. Cell Rep. 43, 113520 (2024).
Kreiman, G., Koch, C. & Fried, I. Category-specific visual responses of single neurons in the human medial temporal lobe. Nat. Neurosci. 3, 946–953 (2000).
Fried, I., MacDonald, K. A. & Wilson, C. L. Single neuron activity in human hippocampus and amygdala during recognition of faces and objects. Neuron 18, 753–765 (1997).
Wang, S. et al. Neurons in the human amygdala selective for perceived emotion. Proc. Natl Acad. Sci. USA 111, E3110–E3119 (2014).
O’keefe, J. & Nadel, L. The Hippocampus as a Cognitive Map (Oxford Univ. Press, 1978).
Behrens, T. E. J. et al. What is a cognitive map? Organizing knowledge for flexible behavior. Neuron 100, 490–509 (2018).
Roweis, S. T. & Saul, L. K. Nonlinear dimensionality reduction by locally linear embedding. Science 290, 2323–2326 (2000).
Tenenbaum, J. B., Silva, V. D. & Langford, J. C. A global geometric framework for nonlinear dimensionality reduction. Science 290, 2319–2323 (2000).
Wang, S. et al. The human amygdala parametrically encodes the intensity of specific facial emotions and their categorical ambiguity. Nat. Commun. 8, 14821 (2017).
Wang, S. et al. Encoding of target detection during visual search by single neurons in the human brain. Curr. Biol. 28, 2058–2069.e4 (2018).
Cao, R. et al. Encoding of facial features by single neurons in the human amygdala and hippocampus. Commun. Biol. 4, 1394 (2021).
Cao, R. et al. A neuronal social trait space for first impressions in the human amygdala and hippocampus. Mol. Psychiatry 27, 3501–3509 (2022).
Liu, Z. et al. Deep learning face attributes in the wild. In Proc. International Conference on Computer Vision (ICCV) 3730–3738 (IEEE, 2015).
Parsons, S., Kruijt, A.-W. & Fox, E. Psychological science needs a standard practice of reporting the reliability of cognitive-behavioral measurements. Adv. Methods Pract. Psychol. Sci. 2, 378–395 (2019).
Grossman, S. et al. Convergent evolution of face spaces across human face-selective neuronal groups and deep convolutional networks. Nat. Commun. 10, 4934 (2019).
Brainard, D. H. The psychophysics toolbox. Spat. Vis. 10, 433–436 (1997).
Parkhi, O. M., Vedaldi, A. & Zisserman, A. Deep Face Recognition. In BMVC 2015—Proceedings of the British Machine Vision Conference 2015 (British Machine Vision Association, 2015).
Hinton, G. E. & Roweis, S. T. Stochastic neighbor embedding. Adv. Neural Inf. Process. Syst. 15, 857–864 (2002).
van der Maaten, L. & Hinton, G. Visualizing data using t-SNE. J. Mach. Learn. Res. 9, 2579–2605 (2008).
Rutishauser, U., Mamelak, A. N. & Schuman, E. M. Single-trial learning of novel stimuli by individual neurons of the human hippocampus-amygdala complex. Neuron 49, 805–813 (2006).
Rutishauser, U. et al. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 464, 903–907 (2010).
Rutishauser, U., Schuman, E. M. & Mamelak, A. N. Online detection and sorting of extracellularly recorded action potentials in human medial temporal lobe recordings, in vivo. J. Neurosci. Methods 154, 204–224 (2006).
Fried, I. et al. Single Neuron Studies of the Human Brain: Probing Cognition (MIT Press, 2014).
Kar, K. et al. Evidence that recurrent circuits are critical to the ventral stream’s execution of core object recognition behavior. Nat. Neurosci. 22, 974–983 (2019).
Kar, K. & DiCarlo, J. J. Fast recurrent processing via ventrolateral prefrontal cortex is needed by the primate ventral stream for robust core visual object recognition. Neuron 109, 164–176.e5 (2021).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. B Stat. Methodol. 57, 289–300 (1995).
Rainer, G., Asaad, W. F. & Miller, E. K. Selective representation of relevant information by neurons in the primate prefrontal cortex. Nature 393, 577–579 (1998).
Minxha, J. et al. Fixations gate species-specific responses to free viewing of faces in the human and macaque amygdala. Cell Rep. 18, 878–891 (2017).
Meyers, E. The neural decoding toolbox. Front. Neuroinform. 7, 8 (2013).
Rutishauser, U. et al. Representation of retrieval confidence by single neurons in the human medial temporal lobe. Nat. Neurosci. 18, 1041–1050 (2015).
Wang, S. et al. Abstract goal representation in visual search by neurons in the human pre-supplementary motor area. Brain 142, 3530–3549 (2019).
Yamins, D. L. K. et al. Performance-optimized hierarchical models predict neural responses in higher visual cortex. Proc. Natl Acad. Sci. USA 111, 8619–8624 (2014).
Kriegeskorte, N., Mur, M. & Bandettini, P. Representational similarity analysis – connecting the branches of systems neuroscience. Front. Syst. Neurosci. 2, 4 (2008).
Mormann, F. et al. Latency and selectivity of single neurons indicate hierarchical processing in the human medial temporal lobe. J. Neurosci. 28, 8865–8872 (2008).
Cao, R. Data for “Feature-based encoding of face identity by single neurons in the human amygdala and hippocampus”. OSF https://doi.org/10.17605/OSF.IO/36KZC (2025).
Acknowledgements
We thank all patients for their participation; staff from WVU Ruby Memorial Hospital for support with patient testing; M. Yin and S. Uddenberg for help with analysis; J. Dawson for contributing the FBI Twins dataset; and R. Adolphs, M. Raichle, C. Ponce, L. Chang, D. Tsao, L. She and P. Webster for discussion and valuable comments. This research was supported by the AFOSR (FA9550-21-1-0088, S.W.), NSF (BCS-1945230, S.W. and X.L.; IIS-2114644, X.L. and S.W.), NIH (K99EY036650, R.C.; R01MH129426, S.W. and X.L.; R01MH120194, J.T.W.; R01EB026439, P.B.; U24NS109103, P.B.; U01NS108916, P.B.; U01NS128612, P.B.; R21NS128307, P.B.; P41EB018783, P.B.), the McDonnell Center for Systems Neuroscience (R.C.), Fondazione Neurone (P.B.), and the Dana Foundation (S.W.). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
R.C., A.T., X.L. and S.W. designed the research. R.C., A.P., P.B. and S.W. performed experiments. N.J.B. and J.T.W. performed surgery. R.C., J.W., C.L., E.D.F., A.P., H.G.R., X.L. and S.W. analysed data. R.C, J.J.D., A.T., U.R., X.L. and S.W. wrote the paper. All authors discussed the results and contributed to the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Human Behaviour thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Results, Discussion, Tables 1 and 2, and Figs. 1–16.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Cao, R., Wang, J., Lin, C. et al. Feature-based encoding of face identity by single neurons in the human amygdala and hippocampus. Nat Hum Behav 9, 1959–1974 (2025). https://doi.org/10.1038/s41562-025-02218-1
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41562-025-02218-1