Abstract
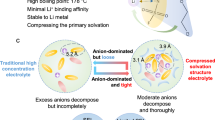
‘Anode-free’ Li metal batteries offer the highest possible energy density but face low Li coulombic efficiency when operated in carbonate electrolytes. Here we report a performance improvement of anode-free Li metal batteries using p-block tin octoate additive in the carbonate electrolyte. We show that the preferential adsorption of the octoate moiety on the Cu substrate induces the construction of a carbonate-less protective layer, which inhibits the side reactions and contributes to the uniform Li plating. In the mean time, the reduction of Sn2+ at the initial charging process builds a stable lithophilic layer of Cu6Sn5 alloy and Sn, improving the affinity between the Li and the Cu substrate. Notably, anode-free Li metal pouch cells with tin octoate additive demonstrate good cycling stability with a high coulombic efficiency of ~99.1%. Furthermore, this in situ p-block layer plating strategy is also demonstrated with other types of p-block metal octoate, as well as a Na metal battery system, demonstrating the high level of universality.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All of the data that support the findings of this study are available in the Article. Atomic configurations of the computational models constructed in this study are available via figshare at https://doi.org/10.6084/m9.figshare.26340670.v1 (ref. 57). Source data are provided with this paper.
References
Armand, M. & Tarascon, J.-M. Building better batteries. Nature 451, 652–657 (2008).
Bruce, P. G., Freunberger, S. A., Hardwick, L. J. & Tarascon, J.-M. Li–O2 and Li–S batteries with high energy storage. Nat. Mater. 11, 19–29 (2012).
Yang, P. & Tarascon, J.-M. Towards systems materials engineering. Nat. Mater. 11, 560–563 (2012).
Tikekar, M. D., Choudhury, S., Tu, Z. & Archer, L. A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 1, 16114 (2016).
Xu, W. et al. Lithium metal anodes for rechargeable batteries. Energy Environ. Sci. 7, 513–537 (2014).
Lu, Y., Tu, Z. & Archer, L. A. Stable lithium electrodeposition in liquid and nanoporous solid electrolytes. Nat. Mater. 13, 961–969 (2014).
Chen, J. et al. Electrolyte design for Li metal-free Li batteries. Mater. Today 39, 118–126 (2020).
Qian, J. et al. Anode-free rechargeable lithium metal batteries. Adv. Funct. Mater. 26, 7094–7102 (2016).
Weber, R. et al. Long cycle life and dendrite-free lithium morphology in anode-free lithium pouch cells enabled by a dual-salt liquid electrolyte. Nat. Energy 4, 683–689 (2019).
Tian, Y. et al. Recently advances and perspectives of anode-free rechargeable batteries. Nano Energy 78, 105344 (2020).
Huang, C. J. et al. Decoupling the origins of irreversible coulombic efficiency in anode-free lithium metal batteries. Nat. Commun. 12, 1452 (2021).
Albertus, P., Babinec, S., Litzelman, S. & Newman, A. Status and challenges in enabling the lithium metal electrode for high-energy and low-cost rechargeable batteries. Nat. Energy 3, 16–21 (2018).
Genovese, M., Louli, A. J., Weber, R., Hames, S. & Dahn, J. R. Measuring the coulombic efficiency of lithium metal cycling in anode-free lithium metal batteries. J. Electrochem. Soc. 165, A3321–A3325 (2018).
Tong, Z., Bazri, B., Hu, S.-F. & Liu, R.-S. Interfacial chemistry in anode-free batteries: challenges and strategies. J. Mater. Chem. A 9, 7396–7406 (2021).
Nanda, S., Gupta, A. & Manthiram, A. Anode-free full cells: a pathway to high-energy density lithium-metal batteries. Adv. Energy Mater. 11, 2000804 (2020).
Beyene, T. T. et al. Concentrated dual-salt electrolyte to stabilize Li metal and increase cycle life of anode free Li-metal batteries. J. Electrochem. Soc. 166, A1501–A1509 (2019).
Yu, Z. et al. Molecular design for electrolyte solvents enabling energy-dense and long-cycling lithium metal batteries. Nat. Energy 5, 526–533 (2020).
Li, S. et al. A multifunctional artificial protective layer for producing an ultra-stable lithium metal anode in a commercial carbonate electrolyte. J. Mater. Chem. A 9, 7667–7674 (2021).
Liu, Y. et al. Solubility-mediated sustained release enabling nitrate additive in carbonate electrolytes for stable lithium metal anode. Nat. Commun. 9, 3656 (2018).
Louli, A. J. et al. Diagnosing and correcting anode-free cell failure via electrolyte and morphological analysis. Nat. Energy 5, 693–702 (2020).
Zheng, J. et al. Electrolyte additive enabled fast charging and stable cycling lithium metal batteries. Nat. Energy 2, 17012 (2017).
Tu, Z. et al. Fast ion transport at solid–solid interfaces in hybrid battery anodes. Nat. Energy 3, 310–316 (2018).
Pathak, R. et al. Fluorinated hybrid solid-electrolyte-interphase for dendrite-free lithium deposition. Nat. Commun. 11, 93 (2020).
Choudhury, S. et al. Electroless formation of hybrid lithium anodes for fast interfacial ion transport. Angew. Chem. Int. Ed. 56, 13070–13077 (2017).
Nayak, P. K., Yang, L., Brehm, W. & Adelhelm, P. From lithium-ion to sodium-ion batteries: advantages, challenges, and surprises. Angew. Chem. Int. Ed. 57, 102–120 (2018).
Soulmi, N. et al. Sn(TFSI)2 as a suitable salt for the electrodeposition of nanostructured Cu6Sn5–Sn composites obtained on a Cu electrode in an ionic liquid. Inorg. Chem. Front. 6, 248–256 (2019).
Biswal, P. et al. The early-stage growth and reversibility of Li electrodeposition in Br-rich electrolytes. Proc. Natl Acad. Sci. USA 118, 2012071118 (2021).
Yan, K. et al. Selective deposition and stable encapsulation of lithium through heterogeneous seeded growth. Nat. Energy 1, 16010 (2016).
Zhang, S. S., Fan, X. & Wang, C. A tin-plated copper substrate for efficient cycling of lithium metal in an anode-free rechargeable lithium battery. Electrochim. Acta 258, 1201–1207 (2017).
Luo, Z. et al. Dendrite-free lithium metal anode with lithiophilic interphase from hierarchical frameworks by tuned nucleation. Energy Storage Mater. 27, 124–132 (2020).
Peled, E., Golodnitsky, D. & Ardel, G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes. J. Electrochem. Soc. 144, L208–L210 (1997).
Moradabadi, A., Bakhtiari, M. & Kaghazchi, P. Effect of anode composition on solid electrolyte interphase formation. Electrochim. Acta 213, 8–13 (2016).
Li, J.-T. et al. XPS and ToF-SIMS study of electrode processes on Sn−Ni alloy anodes for Li-ion batteries. J. Phys. Chem. C 115, 7012–7018 (2011).
Youn, D. H., Heller, A. & Mullins, C. B. Simple synthesis of nanostructured Sn/nitrogen-doped carbon composite using nitrilotriacetic acid as lithium ion battery anode. Chem. Mater. 28, 1343–1347 (2016).
Deng, Z. et al. Ultrasonic scanning to observe wetting and “unwetting” in Li-ion pouch cells. Joule 4, 2017–2029 (2020).
Porion, P. et al. Comparative study on transport properties for LiFAP and LiPF6 in alkyl-carbonates as electrolytes through conductivity, viscosity and NMR self-diffusion measurements. Electrochim. Acta 114, 95–104 (2013).
Gottlieb, H. E., Kotlyar, V. & Nudelman, A. NMR chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 62, 7512–7515 (1997).
Hu, Y.-Y. et al. Origin of additional capacities in metal oxide lithium-ion battery electrodes. Nat. Mater. 12, 1130–1136 (2013).
Tang, M. et al. Following lithiation fronts in paramagnetic electrodes with in situ magnetic resonance spectroscopic imaging. Nat. Commun. 7, 13284 (2016).
Grey, C. P. & Tarascon, J. M. Sustainability and in situ monitoring in battery development. Nat. Mater. 16, 45–56 (2017).
Menkin, S. et al. Toward an understanding of SEI formation and lithium plating on copper in anode-free batteries. J. Phys. Chem. C 125, 16719–16732 (2021).
Frerichs, J. E. et al. 119Sn and 7Li solid-state NMR of the binary Li–Sn intermetallics: structural fingerprinting and impact on the isotropic 119Sn shift via DFT calculations. Chem. Mater. 33, 3499–3514 (2021).
Bhattacharyya, R. et al. In situ NMR observation of the formation of metallic lithium microstructures in lithium batteries. Nat. Mater. 9, 504–510 (2010).
Chandrashekar, S. et al. 7Li MRI of Li batteries reveals location of microstructural lithium. Nat. Mater. 11, 311–315 (2012).
Zhong, Y. et al. Mechanistic insights into fast charging and discharging of the sodium metal battery anode: a comparison with lithium. J. Am. Chem. Soc. 143, 13929–13936 (2021).
Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 54, 11169–11186 (1996).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Frisch, M. J. et al. Gaussian 16, revision C.01 (Gaussian, 2016).
He, X., Zhu, Y., Epstein, A. & Mo, Y. Statistical variances of diffusional properties from ab initio molecular dynamics simulations. npj Comput. Mater. 4, 18 (2018).
Abraham, M. J. et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 1–2, 19–25 (2015).
Wang, J., Wolf, R. M., Caldwell, J. W., Kollman, P. A. & Case, D. A. Development and testing of a general Amber force field. J. Comput. Chem. 25, 1157–1174 (2004).
Zheng, S. et al. VFFDT: a new software for preparing AMBER force field parameters for metal-containing molecular systems. J. Chem. Inf. Model. 56, 811–818 (2016).
Yu, Z. et al. Rational solvent molecule tuning for high-performance lithium metal battery electrolytes. Nat. Energy 7, 94–106 (2022).
Yang, M., Shi, Z., He, Z. & Wang, D. Unraveling electrolyte solvation architectures for high-performance lithium-ion batteries. Sci. China Technol. Sci. 67, 958–964 (2024).
Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Model. Simul. Mater. Sci. Eng. 18, 015012 (2010).
Shi, J. et al. Atomistic configurations of EC and DEC solvents and their related surface models. figshare https://doi.org/10.6084/m9.figshare.26340670.v1 (2024).
Acknowledgements
The work leading to these results received funding from the National Natural Science Foundation of China (22179098, to J.M.) and the Fundamental Research Funds for the Central Universities. L.Q. acknowledges support from the National Natural Science Foundation of China (52272203). Y.H. acknowledges support from the National Natural Science Foundation of China (52027816). M.T. acknowledges support from the National Natural Science Foundation of China (22090043). M.Y. acknowledges support from the National Natural Science Foundation of China (52302302). P.S. and T.K. acknowledge support from the German Research Foundation (STR 596/13−1).
Author information
Authors and Affiliations
Contributions
J.M. conceived and coordinated the project. J.S., T.K., Z.Z., L.S., J.L., M.T., Z.D., M.L., J.X., Y.S., L.Q., Y.H., P.S. and J.M. carried out experimental work and data analysis. M.Y. performed theoretical calculations. All authors discussed the results. J.S., T.K., Z.Z., M.Y., L.Q., Y.H., P.S. and J.M. wrote the paper with the contributions of all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Stefan Freunberger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–28 and Tables 1 and 2.
Source data
Source Data Fig. 1
Electrochemical stability of metal anode with and without additive.
Source Data Fig. 2
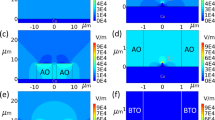
Regulation of Sn(Oct)2 additive on the Li plating/stripping.
Source Data Fig. 3
Li plating/stripping behaviour and the characterization of plated Li.
Source Data Fig. 4
Electrochemical performance of Li || NCM coin cells and Cu || NCM pouch cells, cell visualization and Li+ solvation analysis.
Source Data Fig. 5
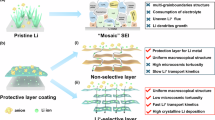
Mechanism of Sn(Oct)2 additive on Li plating process.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shi, J., Koketsu, T., Zhu, Z. et al. In situ p-block protective layer plating in carbonate-based electrolytes enables stable cell cycling in anode-free lithium batteries. Nat. Mater. 23, 1686–1694 (2024). https://doi.org/10.1038/s41563-024-01997-8
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41563-024-01997-8
This article is cited by
-
Dual-gradient metal layer for practicalizing high-energy lithium batteries
Nature Communications (2025)