Abstract
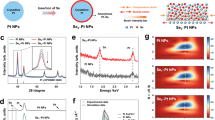
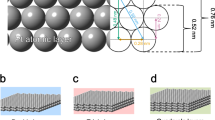
PtM (M = S, Se, Te) dichalcogenides are promising two-dimensional materials for electronics, optoelectronics and gas sensors due to their high air stability, tunable bandgap and high carrier mobility. However, their potential as electrocatalysts for the oxygen reduction reaction (ORR) is often underestimated due to their semiconducting properties and limited surface area from van der Waals stacking. Here we show an approach for synthesizing a highly efficient and stable ORR catalyst by restructuring defective platinum diselenide (DEF-PtSe2) through electrochemical cycling in an O2-saturated electrolyte. After 42,000 cycles, DEF-PtSe2 exhibited 1.3 times higher specific activity and 2.6 times higher mass activity compared with a commercial Pt/C electrocatalyst. Even after 126,000 cycles, it maintained superior ORR performance with minimal decay. Quantum mechanical calculations using hybrid density functional theory reveal that the improved performance is due to the synergistic contributions from Pt nanoparticles and the apical active sites on the DEF-PtSe2 surface. This work highlights the potential of DEF-PtSe2 as a durable electrocatalyst for ORR, offering insights into PtM dichalcogenide electrochemistry and the design of advanced catalysts.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data that support the findings of this study are available in the Supplementary Information.
References
Shao, M., Chang, Q., Dodelet, J. P. & Chenitz, R. Recent advances in electrocatalysts for oxygen reduction reaction. Chem. Rev. 116, 3594–3657 (2016).
Dey, S. et al. Molecular electrocatalysts for the oxygen reduction reaction. Nat. Rev. Chem. 1, 0098 (2017).
Kulkarni, A., Siahrostami, S., Patel, A. & Nørskov, J. K. Understanding catalytic activity trends in the oxygen reduction reaction. Chem. Rev. 118, 2302–2312 (2018).
Bu, L. et al. PtPb/PtNi intermetallic core/atomic layer shell octahedra for efficient oxygen reduction electrocatalysis. J. Am. Chem. Soc. 139, 9576–9582 (2017).
Strasser, P. & Kühl, S. Dealloyed Pt-based core-shell oxygen reduction electrocatalysts. Nano Energy 29, 166–177 (2016).
Wang, X. et al. Review of metal catalysts for oxygen reduction reaction: from nanoscale engineering to atomic design. Chem 5, 1486–1511 (2019).
Lin, R. et al. High-performance graphene/β-Ga2O3 heterojunction deep-ultraviolet photodetector with hot-electron excited carrier multiplication. ACS Appl. Mater. Interfaces 10, 22419–22426 (2018).
Luo, M. et al. Stable high‐index faceted Pt skin on zigzag‐like PtFe nanowires enhances oxygen reduction catalysis. Adv. Mater. 30, 1705515 (2018).
Jiang, K. et al. Efficient oxygen reduction catalysis by subnanometer Pt alloy nanowires. Sci. Adv. 3, e1601705 (2017).
Najam, T. et al. An efficient anti‐poisoning catalyst against SOx, NOx, and POx: P, N‐doped carbon for oxygen reduction in acidic media. Angew. Chem. Int. Ed. 130, 15321–15326 (2018).
Wang, Y. et al. Monolayer PtSe2, a new semiconducting transition-metal-dichalcogenide, epitaxially grown by direct selenization of Pt. Nano Lett. 15, 4013–4018 (2015).
Wagner, S. et al. Highly sensitive electromechanical piezoresistive pressure sensors based on large-area layered PtSe2 films. Nano Lett. 18, 3738–3745 (2018).
Hu, D. et al. Unveiling the layer‐dependent catalytic activity of PtSe2 atomic crystals for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 131, 7051–7055 (2019).
Wang, Y., Li, Y. & Heine, T. PtTe monolayer: two-dimensional electrocatalyst with high basal plane activity toward oxygen reduction reaction. J. Am. Chem. Soc. 140, 12732–12735 (2018).
Lin, S. et al. Tunable active edge sites in PtSe2 films towards hydrogen evolution reaction. Nano Energy 42, 26–33 (2017).
Chia, X. et al. Layered platinum dichalcogenides (PtS2, PtSe2, and PtTe2) electrocatalysis: monotonic dependence on the chalcogen size. Adv. Funct. Mater. 26, 4306–4318 (2016).
Wang, Z. et al. A noble metal dichalcogenide for high‐performance field‐effect transistors and broadband photodetectors. Adv. Funct. Mater. 30, 1907945 (2020).
Cao, K. et al. Nanofence stabilized platinum nanoparticles catalyst via facet‐selective atomic layer deposition. Small 13, 1700648 (2017).
Durst, J. et al. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 7, 2255–2260 (2014).
Lucas, C. A., Markovic, N. M. & Ross, P. N. Underpotential deposition of Cu on Pt (001): interface structure and the influence of adsorbed bromide. Phys. Rev. B 57, 13184 (1998).
Green, C. L. & Kucernak, A. Determination of the platinum and ruthenium surface areas in platinum–ruthenium alloy electrocatalysts by underpotential deposition of copper. I. Unsupported catalysts. J. Phys. Chem. B 106, 1036–1047 (2002).
Liu, P., Ge, X., Wang, R., Ma, H. & Ding, Y. Facile fabrication of ultrathin Pt overlayers onto nanoporous metal membranes via repeated Cu UPD and in situ redox replacement reaction. Langmuir 25, 561–567 (2009).
Becke, A. D. A new mixing of Hartree-Fock and local density-functional theories. J. Chem. Phys. 98, 1372–1377 (1993).
Heyd, J., Peralta, J. E., Scuseria, G. E. & Martin, R. L. Energy band gaps and lattice parameters evaluated with the Heyd-Scuseria-Ernzerhof screened hybrid functional. J. Chem. Phys. 123, 174101 (2005).
Lei, Y. et al. Low-temperature synthesis of heterostructures of transition metal dichalcogenide alloys (WxMo1–xS2) and graphene with superior catalytic performance for hydrogen evolution. ACS Nano 11, 5103 (2017).
Parac, M., Etinski, M., Peric, M. & Grimme, S. A theoretical investigation of the geometries and binding energies of molecular tweezer and clip host−guest systems. J. Chem. Theory Comput. 1, 1110–1118 (2005).
Han, S. S. et al. Horizontal-to-vertical transition of 2D layer orientation in low-temperature chemical vapor deposition-grown PtSe2 and its influences on electrical properties and device applications. ACS Appl. Mater. Interfaces 11, 13598–13607 (2019).
Dong, J. C. et al. In situ Raman spectroscopic evidence for oxygen reduction reaction intermediates at platinum single-crystal surfaces. Nat. Energy 4, 60–67 (2019).
Niu, W. et al. Mesoporous N-doped carbons prepared with thermally removable nanoparticle templates: an efficient electrocatalyst for oxygen reduction reaction. J. Am. Chem. Soc. 137, 5555–5562 (2015).
Bai, G. et al. Atomic carbon layers supported Pt nanoparticles for minimized CO poisoning and maximized methanol oxidation. Small 15, 1902951 (2019).
Wang, P. et al. Ternary Pt9RhFex nanoscale alloys as highly efficient catalysts with enhanced activity and excellent CO-poisoning tolerance for ethanol oxidation. ACS Appl. Mater. Interfaces 9, 9584–9591 (2017).
Hersbach, T. J., Ye, C., Garcia, A. C. & Koper, M. T. Tailoring the electrocatalytic activity and selectivity of Pt (111) through cathodic corrosion. ACS Catal. 10, 15104–15113 (2020).
Li, K. et al. The oxygen reduction reaction on Pt (111) and Pt (100) surfaces substituted by subsurface Cu: a theoretical perspective. J. Mater. Chem. A 3, 11444–11452 (2015).
Liu, S., White, M. G. & Liu, P. Mechanism of oxygen reduction reaction on Pt (111) in alkaline solution: importance of chemisorbed water on surface. J. Phys. Chem. C 120, 15288–15298 (2016).
Viswanathan, V., Hansen, H. A., Rossmeisl, J. & Nørskov, J. K. Unifying the 2e– and 4e– reduction of oxygen on metal surfaces. J. Phys. Chem. Lett. 3, 2948–2951 (2012).
Acknowledgements
B.E.K. and W.N. were financially supported by the National Science Foundation (NSF) under grant no. CHE-1800376. B.E.K. and W.N. also acknowledge the use of Princeton’s Imaging and Analysis Center, which is partially supported through the Princeton Center for Complex Materials (PCCM), an NSF-MRSEC program (DMR-2011750). F.Z. acknowledges financial support from the Simons Foundation (no. 377485) and John Templeton Foundation (no. 58851). S.P. acknowledges financial support from the Science and Engineering Research Board, under the schemes Core Research Grant (no. CRG/2021/000572); ECRA (no. ECR/2018/000255); and Council of Scientific & Industrial Research (no. 22/0883/23/EMR-II). B.E.K. and W.N. thank M. R. Smith for his helpful comments and careful proofreading of the manuscript. J.L.M.-C. acknowledges startup funds from Michigan State University. The computational and theoretical calculations presented in this work were supported in part through computational resources and services provided by the Institute for Cyber-Enabled Research at Michigan State University.
Author information
Authors and Affiliations
Contributions
The manuscript was written through contributions of all authors. W.N., S.P., J.L.M.-C. and B.E.K. developed the ideas and directed the experiments and theoretical calculations. W.N. and F.Z. synthesized the materials. G.C. and N.Y. conducted the STEM measurements for this work. J.L.M.-C. designed and directed the theoretical calculations. S.P. carried out most of the DFT calculations. J.L.M.-C. led the submission and resubmission efforts, coordinated responses from all authors throughout the review process, and is acknowledged for shouldering this important task with perseverance over the course of this multi-year project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Fernando Soto and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–16, Tables 1–5 and discussion.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niu, W., Pakhira, S., Cheng, G. et al. Reaction-driven restructuring of defective PtSe2 into ultrastable catalyst for the oxygen reduction reaction. Nat. Mater. 23, 1704–1711 (2024). https://doi.org/10.1038/s41563-024-02020-w
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41563-024-02020-w
This article is cited by
-
Asymmetric Pt1C3-Pt1O1C3 catalytic pairs for efficient transfer hydrogenation of azobenzene
Nature Communications (2026)
-
Interfacial electric field engineering in Ni-ZrC heterostructures for high-efficiency zinc-air batteries
Science China Chemistry (2026)
-
Hydrogen Production, Storage, and Decarbonization via Heterogenous Catalytic CO2 Hydrogenation
Korean Journal of Chemical Engineering (2025)
-
Interfacial properties of monolayer PtSe2 FETs: A comparative study of traditional metal and semimetal electrodes
Applied Physics A (2025)