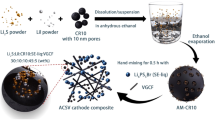
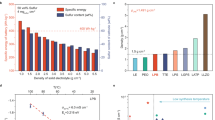
Abstract
Lithium–sulfur (Li–S) all-solid-state batteries (ASSBs) hold great promise for next-generation safe, durable and energy-dense battery technology. However, solid-state sulfur conversion reactions are kinetically sluggish and primarily constrained to the restricted three-phase boundary area of sulfur, carbon and solid electrolytes, making it challenging to achieve high sulfur utilization. Here we develop and implement mixed ionic–electronic conductors (MIECs) in sulfur cathodes to replace conventional solid electrolytes and invoke conversion reactions at sulfur–MIEC interfaces in addition to traditional three-phase boundaries. Microscopic and tomographic analyses reveal the emergence of mixed-conducting domains embedded in sulfur at sulfur–MIEC boundaries, helping promote the thorough conversion of active sulfur into Li2S. Consequently, substantially improved active sulfur ratios (up to 87.3%) and conversion degrees (>94%) are achieved in Li–S ASSBs with high discharge capacity (>1,450 mAh g–1) and long cycle life (>1,000 cycles). The strategy is also applied to enhance the active material utilization of other conversion cathodes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Computational data are available via figshare at https://doi.org/10.6084/m9.figshare.27257931 (ref. 55).
References
Manthiram, A., Yu, X. & Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2, 16103 (2017).
Tan, D. H. S. et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 373, 1494–1499 (2021).
Xiao, Y. et al. Electrolyte melt infiltration for scalable manufacturing of inorganic all-solid-state lithium-ion batteries. Nat. Mater. 20, 984–990 (2021).
Kamaya, N. et al. A lithium superionic conductor. Nat. Mater. 10, 682–686 (2011).
Alexander, G. V., Shi, C., O’Neill, J. & Wachsman, E. D. Extreme lithium-metal cycling enabled by a mixed ion- and electron-conducting garnet three-dimensional architecture. Nat. Mater. 22, 1136–1143 (2023).
Jun, K. et al. Lithium superionic conductors with corner-sharing frameworks. Nat. Mater. 21, 924–931 (2022).
Ji, X., Lee, K. T. & Nazar, L. F. A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries. Nat. Mater. 8, 500–506 (2009).
Zhao, C. et al. A high-energy and long-cycling lithium–sulfur pouch cell via a macroporous catalytic cathode with double-end binding sites. Nat. Nanotechnol. 16, 166–173 (2021).
Li, Z. et al. Lithiated metallic molybdenum disulfide nanosheets for high-performance lithium–sulfur batteries. Nat. Energy 8, 84–93 (2023).
Huang, Q. et al. Cycle stability of conversion-type iron fluoride lithium battery cathode at elevated temperatures in polymer electrolyte composites. Nat. Mater. 18, 1343–1349 (2019).
Hua, X. et al. Revisiting metal fluorides as lithium-ion battery cathodes. Nat. Mater. 20, 841–850 (2021).
Chung, W. J. et al. The use of elemental sulfur as an alternative feedstock for polymeric materials. Nat. Chem. 5, 518–524 (2013).
Muench, S. et al. Polymer-based organic batteries. Chem. Rev. 116, 9438–9484 (2016).
Wu, F. & Yushin, G. Conversion cathodes for rechargeable lithium and lithium-ion batteries. Energy Environ. Sci. 10, 435–459 (2017).
Janek, J. & Zeier, W. G. Challenges in speeding up solid-state battery development. Nat. Energy 8, 230–240 (2023).
Zhang, J. et al. Microstructure engineering of solid-state composite cathode via solvent-assisted processing. Joule 5, 1845–1859 (2021).
Manthiram, A., Fu, Y., Chung, S.-H., Zu, C. & Su, Y.-S. Rechargeable lithium–sulfur batteries. Chem. Rev. 114, 11751–11787 (2014).
Bradbury, R. et al. Visualizing reaction fronts and transport limitations in solid-state Li–S batteries via operando neutron imaging. Adv. Energy Mater. 13, 2203426 (2023).
Ohno, S., Rosenbach, C., Dewald, G. F., Janek, J. & Zeier, W. G. Linking solid electrolyte degradation to charge carrier transport in the thiophosphate-based composite cathode toward solid-state lithium-sulfur batteries. Adv. Funct. Mater. 31, 2010620 (2021).
Zhou, J. et al. Healable and conductive sulfur iodide for solid-state Li–S batteries. Nature 627, 301–305 (2024).
Han, F. et al. High-performance all-solid-state lithium–sulfur battery enabled by a mixed-conductive Li2S nanocomposite. Nano Lett. 16, 4521–4527 (2016).
Yao, X. et al. High-performance all-solid-state lithium–sulfur batteries enabled by amorphous sulfur-coated reduced graphene oxide cathodes. Adv. Energy Mater. 7, 1602923 (2017).
Zhang, Y. et al. High-performance all-solid-state lithium–sulfur batteries with sulfur/carbon nano-hybrids in a composite cathode. J. Mater. Chem. A 6, 23345–23356 (2018).
Hou, L.-P. et al. Improved interfacial electronic contacts powering high sulfur utilization in all-solid-state lithium–sulfur batteries. Energy Storage Mater. 25, 436–442 (2020).
Sakuda, A., Sato, Y., Hayashi, A. & Tatsumisago, M. Sulfur-based composite electrode with interconnected mesoporous carbon for all-solid-state lithium–sulfur batteries. Energy Technol. 7, 1900077 (2019).
Shi, X. et al. Fast Li-ion conductor of Li3HoBr6 for stable all-solid-state lithium–sulfur battery. Nano Lett. 21, 9325–9331 (2021).
Wang, D. et al. Realizing high-capacity all-solid-state lithium-sulfur batteries using a low-density inorganic solid-state electrolyte. Nat. Commun. 14, 1895 (2023).
Kwok, C. Y., Xu, S., Kochetkov, I., Zhou, L. & Nazar, L. F. High-performance all-solid-state Li2S batteries using an interfacial redox mediator. Energy Environ. Sci. 16, 610–618 (2023).
Xu, S. et al. A high capacity all solid-state Li–sulfur battery enabled by conversion–intercalation hybrid cathode architecture. Adv. Funct. Mater. 31, 2004239 (2021).
Ulissi, U. et al. High capacity all-solid-state lithium batteries enabled by pyrite–sulfur composites. Adv. Energy Mater. 8, 1801462 (2018).
Alzahrani, A. S. et al. Confining sulfur in porous carbon by vapor deposition to achieve high-performance cathode for all-solid-state lithium–sulfur batteries. ACS Energy Lett. 6, 413–418 (2021).
Kim, H., Choi, H.-N., Hwang, J.-Y., Yoon, C. S. & Sun, Y.-K. Tailoring the interface between sulfur and sulfide solid electrolyte for high-areal-capacity all-solid-state lithium–sulfur batteries. ACS Energy Lett. 8, 3971–3979 (2023).
Zhou, G., Chen, H. & Cui, Y. Formulating energy density for designing practical lithium–sulfur batteries. Nat. Energy 7, 312–319 (2022).
Cao, D. et al. Understanding electrochemical reaction mechanisms of sulfur in all-solid-state batteries through operando and theoretical studies. Angew. Chem. Int. Ed. 135, e202302363 (2023).
Xiao, Y. et al. Comparison of sulfur cathode reactions between a concentrated liquid electrolyte system and a solid-state electrolyte system by soft X-ray absorption spectroscopy. ACS Appl. Energy Mater. 4, 186–193 (2021).
Dietrich, C. et al. Lithium ion conductivity in Li2S–P2S5 glasses—building units and local structure evolution during the crystallization of superionic conductors Li3PS4, Li7P3S11 and Li4P2S7. J. Mater. Chem. A 5, 18111–18119 (2017).
Sun, K. et al. Interaction of TiS2 and sulfur in Li–S battery system. J. Electrochem. Soc. 164, A1291–A1297 (2017).
Dewald, G. F., Ohno, S., Hering, J. G., Janek, J. & Zeier, W. G. Analysis of charge carrier transport toward optimized cathode composites for all‐solid‐state Li–S batteries. Batteries Supercaps 4, 183–194 (2021).
Han, F. et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4, 187–196 (2019).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Schwietert, T. K. et al. Clarifying the relationship between redox activity and electrochemical stability in solid electrolytes. Nat. Mater. 19, 428–435 (2020).
Notestein, J. M. et al. Structural assessment and catalytic Consequences of the oxygen coordination environment in grafted Ti−calixarenes. J. Am. Chem. Soc. 129, 1122–1131 (2007).
Cutsail, G. E. III & DeBeer, S. Challenges and opportunities for applications of advanced X-ray spectroscopy in catalysis research. ACS Catal. 12, 5864–5886 (2022).
Wang, Y. et al. Superionic conduction and interfacial properties of the low temperature phase Li7P2S8Br0.5I0.5. Energy Storage Mater. 19, 80–87 (2019).
Ravel, B. & Newville, M. ATHENA, ARTEMIS, HEPHAESTUS: data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 12, 537–541 (2005).
Singh, V., Kosa, M., Majhi, K. & Major, D. T. Putting DFT to the test: a first-principles study of electronic, magnetic, and optical properties of Co3O4. J. Chem. Theory Comput. 11, 64–72 (2015).
Kresse, G. & Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 59, 1758–1775 (1999).
Dronskowski, R. & Bloechl, P. E. Crystal orbital Hamilton populations (COHP): energy-resolved visualization of chemical bonding in solids based on density-functional calculations. J. Phys. Chem. 97, 8617–8624 (1993).
Deringer, V. L., Tchougréeff, A. L. & Dronskowski, R. Crystal orbital Hamilton population (COHP) analysis as projected from plane–wave basis sets. J. Phys. Chem. A 115, 5461–5466 (2011).
Maintz, S., Deringer, V. L., Tchougréeff, A. L. & Dronskowski, R. Analytic projection from plane–wave and PAW wavefunctions and application to chemical-bonding analysis in solids. J. Comput. Chem. 34, 2557–2567 (2013).
Peng, J., Wang, X., Li, H., Chen, L. & Wu, F. High-capacity, long-life iron fluoride all-solid-state lithium battery with sulfide solid electrolyte. Adv. Energy Mater. 13, 2300706 (2023).
Wan, T. H., Saccoccio, M., Chen, C. & Ciucci, F. Influence of the discretization methods on the distribution of relaxation times deconvolution: implementing radial basis functions with DRTtools. Electrochim. Acta 184, 483–499 (2015).
Wang, D. et al. Overcoming the conversion reaction limitation at three-phase interfaces using mixed conductors towards energy-dense solid-state Li–S batteries. figshare https://doi.org/10.6084/m9.figshare.27257931 (2024).
Acknowledgements
This work was supported by the Assistant Secretary for Energy Efficiency and Renewable Energy, Office of Vehicle Technologies of the US Department of Energy, through Advanced Battery Materials Research (BMR) program award number DE-EE0008862 (Da. Wang, L.-J.J., R.K. and Do. Wang) and Battelle-Pacific Northwest National Laboratory subcontract award 680628 (Battery500 Consortium, Da. Wang, L.-J.J., L.Y., A.S., A.T.N. and Do. Wang). We gratefully acknowledge the computing resources provided on Bebop, a high-performance computing cluster operated by the Laboratory Computing Resource Center at Argonne National Laboratory. This research used 8-BM of the National Synchrotron Light Source II, a US Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract number DE-SC0012704 (D. Wierzbicki and Y.D.). The APT characterization performed at EMSL, a DOE Office of Science User Facility sponsored by the Office of Biological and Environmental Research, was supported by the US DOE Office of Electricity (OE) under contract DE-AC05-76RL01830 (B.G. and X.L.) through Pacific Northwest National Laboratory project number 70247 (Long Duration and Cost Competitive Energy Storage). In addition, we thank C. George, J. L. Gray and J. Meyet from the Pennsylvania State University for their instrument assistance.
Author information
Authors and Affiliations
Contributions
Da. Wang and Do. Wang conceived the idea and designed the experiments. B.G. and X.L. conducted the APT experiments. D.Wierzbicki and Y.D. carried out the XAS characterization and analysis. Da. Wang., L.-J.J., A.S. and L.Y. prepared the materials, assembled the batteries and conducted electrochemical tests. V.S., T.R. and A.T.N. designed and performed the simulation. Da. Wang carried out the TEM experiments. M.L. and H.J. conducted the XRD analysis. Da. Wang and R.K. performed the SEM tests. Da. Wang performed the quantitative analysis of dead sulfur. S.S. and Da. Wang designed the Swagelok cell for electrochemical testing. Da. Wang, Do. Wang and X.L. wrote the paper with comments from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Yan Yao and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–39, Tables 1–11 and Notes 1–8.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, D., Gwalani, B., Wierzbicki, D. et al. Overcoming the conversion reaction limitation at three-phase interfaces using mixed conductors towards energy-dense solid-state Li–S batteries. Nat. Mater. 24, 243–251 (2025). https://doi.org/10.1038/s41563-024-02057-x
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41563-024-02057-x
This article is cited by
-
W/V Dual-Atom Doping MoS2-Mediated Phase Transition for Efficient Polysulfide Adsorption/Conversion Kinetics in Lithium–Sulfur Battery
Nano-Micro Letters (2026)
-
Metal-organic frameworks derived single atom catalysts for lithium sulfur batteries
Communications Materials (2025)
-
Architected continuum mixed ionic and electronic conducting alloy negative electrode for fast-charging all-solid-state lithium batteries
Nature Communications (2025)
-
Assessing the practical feasibility of solid-state lithium–sulfur batteries
Communications Materials (2025)
-
Suppression strategies for the polysulfide shuttle effect in electrolyte systems
Communications Materials (2025)