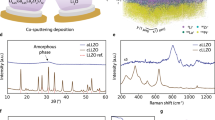
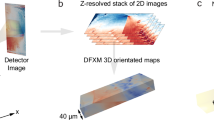
Abstract
All-solid-state batteries offer high-energy-density and eco-friendly energy storage but face commercial hurdles due to dendrite formation, especially with lithium metal anodes. Here we report that dendrite formation in Li/Li7La3Zr2O12/Li batteries occurs via two distinct mechanisms, using non-invasive solid-state nuclear magnetic resonance and magnetic resonance imaging. Tracer-exchange nuclear magnetic resonance shows non-uniform Li plating at electrode–electrolyte interfaces and local Li+ reduction at Li7La3Zr2O12 grain boundaries. In situ magnetic resonance imaging reveals rapid dendrite formation via non-uniform Li plating, followed by sluggish bulk dendrite nucleation from Li+ reduction, with an intervening period of stalled growth. Formation of amorphous dendrites and subsequent crystallization, the defect chemistry of solid electrolytes and battery operating conditions play a critical role in shaping the complex interplay between the two mechanisms. Overall, this work deepens our understanding of dendrite formation in solid-state Li batteries and provides comprehensive insight that might be valuable for mitigating dendrite-related challenges.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Data generated or analysed during this study are also included in Supplementary Information. Further data are available from the corresponding authors upon request. Source data are provided with this paper.
Code availability
The Python code used for simulating the tracer-exchange process is provided via GitHub at https://github.com/Jak1022/Python_tracer_exchange.
References
Harry, K. J., Hallinan, D. T., Parkinson, D. Y., MacDowell, A. A. & Balsara, N. P. Detection of subsurface structures underneath dendrites formed on cycled lithium metal electrodes. Nat. Mater. 13, 69–73 (2014).
Cheng, E. J., Sharafi, A. & Sakamoto, J. Intergranular Li metal propagation through polycrystalline Li6.25Al0.25La3Zr2O12 ceramic electrolyte. Electrochim. Acta 223, 85–91 (2017).
Porz, L. et al. Mechanism of lithium metal penetration through inorganic solid electrolytes. Adv. Energy Mater. 7, 1701003 (2017).
Krauskopf, T. et al. Lithium-metal growth kinetics on LLZO garnet-type solid electrolytes. Joule 3, 2030–2049 (2019).
Manalastas, W. et al. Mechanical failure of garnet electrolytes during Li electrodeposition observed by in-operando microscopy. J. Power Sources 412, 287–293 (2019).
Westover, A. S., Dudney, N. J., Sacci, R. L. & Kalnaus, S. Deposition and confinement of Li metal along an artificial Lipon–Lipon interface. ACS Energy Lett. 4, 651–655 (2019).
Kazyak, E. et al. Li penetration in ceramic solid electrolytes: operando microscopy analysis of morphology, propagation, and reversibility. Matter 2, 1025–1048 (2020).
Ning, Z. et al. Visualizing plating-induced cracking in lithium-anode solid-electrolyte cells. Nat. Mater. 20, 1121–1129 (2021).
Lv, S. et al. Operando monitoring the lithium spatial distribution of lithium metal anodes. Nat. Commun. 9, 2152 (2018).
Ning, Z. et al. Dendrite initiation and propagation in lithium metal solid-state batteries. Nature 618, 287–293 (2023).
Krauskopf, T., Hartmann, H., Zeier, W. G. & Janek, J. Toward a fundamental understanding of the lithium metal anode in solid-state batteries—an electrochemo-mechanical study on the garnet-type solid electrolyte Li6.25Al0.25La3Zr2O12. ACS Appl. Mater. Interfaces 11, 14463–14477 (2019).
Tsai, C.-L. et al. Li7La3Zr2O12 interface modification for Li dendrite prevention. ACS Appl. Mater. Interfaces 8, 10617–10626 (2016).
Heo, S. et al. Short-circuit mechanism induced by crack propagation spurred by inhomogeneous electric field in garnet-based solid electrolyte. J. Power Sources 510, 230389 (2021).
Tian, H.-K., Xu, B. & Qi, Y. Computational study of lithium nucleation tendency in Li7La3Zr2O12 (LLZO) and rational design of interlayer materials to prevent lithium dendrites. J. Power Sources 392, 79–86 (2018).
Tian, H.-K., Liu, Z., Ji, Y., Chen, L.-Q. & Qi, Y. Interfacial electronic properties dictate Li dendrite growth in solid electrolytes. Chem. Mater. 31, 7351–7359 (2019).
Zhao, C.-Z. et al. An ion redistributor for dendrite-free lithium metal anodes. Sci. Adv. 4, eaat3446 (2018).
Lin, D. et al. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 11, 626–632 (2016).
Song, Y. et al. Revealing the short‐circuiting mechanism of garnet‐based solid‐state electrolyte. Adv. Energy Mater. 9, 1900671 (2019).
Liu, X. et al. Local electronic structure variation resulting in Li ‘filament’ formation within solid electrolytes. Nat. Mater. 20, 1485–1490 (2021).
Gao, H. et al. Visualizing the failure of solid electrolyte under GPa-level interface stress induced by lithium eruption. Nat. Commun. 13, 5050 (2022).
Ye, L. & Li, X. A dynamic stability design strategy for lithium metal solid state batteries. Nature 593, 218–222 (2021).
Zhu, C. et al. Understanding the evolution of lithium dendrites at Li6.25Al0.25La3Zr2O12 grain boundaries via operando microscopy techniques. Nat. Commun. 14, 1300 (2023).
Han, F. et al. High electronic conductivity as the origin of lithium dendrite formation within solid electrolytes. Nat. Energy 4, 187–196 (2019).
Ping, W. et al. Reversible short‐circuit behaviors in garnet‐based solid‐state batteries. Adv. Energy Mater. 10, 2000702 (2020).
Li, Q. et al. In-situ visualization of lithium plating in all-solid-state lithium-metal battery. Nano Energy 63, 103895 (2019).
Song, B. et al. Dynamic lithium distribution upon dendrite growth and shorting revealed by operando neutron imaging. ACS Energy Lett. 4, 2402–2408 (2019).
Murugan, R., Thangadurai, V. & Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 46, 7778–7781 (2007).
Richards, W. D., Miara, L. J., Wang, Y., Kim, J. C. & Ceder, G. Interface stability in solid-state batteries. Chem. Mater. 28, 266–273 (2016).
Binninger, T., Marcolongo, A., Mottet, M., Weber, V. & Laino, T. Comparison of computational methods for the electrochemical stability window of solid-state electrolyte materials. J. Mater. Chem. A 8, 1347–1359 (2020).
Wandt, J. et al. Operando electron paramagnetic resonance spectroscopy – formation of mossy lithium on lithium anodes during charge–discharge cycling. Energy Environ. Sci. 8, 1358–1367 (2015).
Niemöller, A., Jakes, P., Eichel, R.-A. & Granwehr, J. EPR imaging of metallic lithium and its application to dendrite localisation in battery separators. Sci. Rep. 8, 14331 (2018).
Fang, Q. & Lafdi, K. Effect of nanofiller morphology on the electrical conductivity of polymer nanocomposites. Nano Express 2, 010019 (2021).
Hayamizu, K., Terada, Y., Kataoka, K., Akimoto, J. & Haishi, T. Relationship between Li+ diffusion and ion conduction for single-crystal and powder garnet-type electrolytes studied by 7Li PGSE NMR spectroscopy. Phys. Chem. Chem. Phys. 21, 23589–23597 (2019).
Lewis, J. A. et al. Linking void and interphase evolution to electrochemistry in solid-state batteries using operando X-ray tomography. Nat. Mater. 20, 503–510 (2021).
Wang, H., Lin, D., Liu, Y., Li, Y. & Cui, Y. Ultrahigh–current density anodes with interconnected Li metal reservoir through overlithiation of mesoporous AlF3 framework. Sci. Adv. 3, e1701301 (2017).
Chien, P.-H. et al. Li distribution heterogeneity in solid electrolyte Li10GeP2S12 upon electrochemical cycling probed by 7Li MRI. J. Phys. Chem. Lett. 9, 1990–1998 (2018).
Yang, M., Liu, Y. & Mo, Y. Lithium crystallization at solid interfaces. Nat. Commun. 14, 2986 (2023).
Yang, M., Liu, Y., Nolan, A. M. & Mo, Y. Interfacial atomistic mechanisms of lithium metal stripping and plating in solid‐state batteries. Adv. Mater. 33, 2008081 (2021).
Haase, A. Snapshot FLASH MRI. Applications to T1, T2, and chemical-shift imaging. Magn. Reson. Med. 13, 77–89 (1990).
Serša, I. & Mikac, U. A study of MR signal reception from a model for a battery cell. J. Magn. Reson. 294, 7–15 (2018).
Acknowledgements
We acknowledge the support from the National Science Foundation under grant no. DMR-2319151 for Y.C. and Y.-Y.H. All solid-state NMR experiments were performed at the National High Magnetic Field Laboratory, which is supported by National Science Foundation Cooperative Agreement nos DMR-1644779 and DMR-2128556. We thank the US Department of Energy (DOE), Office of Science, Office of Basic Energy Sciences programme for support under award no. DE-SC0019121 for D.H. and H.X. This research used resources of the Center for Nanoscale Materials, a DOE Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under contract DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
Y.-Y.H. conceived the concept, designed the experiments and supervised the project. S.C.G. directed and supervised the MRI component of the study. H.L., Y.C. and P.-H.C. performed LLZO synthesis, X-ray diffraction characterization, symmetrical cell assembly and electrochemical measurements. H.L., Y.C., P.-H.C. and E.T. carried out 6,7Li NMR. H.L. and Y.C. performed numerical simulations and Li isotope composition analysis of cycled samples. S.C.G., J.T.R., H.L., Y.C., P.-H.C., G.A., I.P.O., J.B., S.W.H. and Y.-Y.H. performed MRI and data analysis. P.L.G. developed the MRI gradient coil for in situ 2D MRI. D.H. and H.X. contributed to TEM and energy-filtered TEM data acquisition and analysis. Y.-Y.H., H.L., Y.C. and P.-H.C. wrote the paper with contributions from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–10 and Scheme 1.
Source data
Source Data Fig. 1
Cycling voltage profile, solid-state NMR and EPR data.
Source Data Fig. 2
The solid-state NMR and material composition data.
Source Data Fig. 4
Cycling voltage profile and dendrite formation data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, H., Chen, Y., Chien, PH. et al. Dendrite formation in solid-state batteries arising from lithium plating and electrolyte reduction. Nat. Mater. 24, 581–588 (2025). https://doi.org/10.1038/s41563-024-02094-6
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41563-024-02094-6
This article is cited by
-
Deciphering lithium penetration through solids
Nature Materials (2025)
-
Grain boundary amorphization as a strategy to mitigate lithium dendrite growth in solid-state batteries
Nature Communications (2025)