Abstract
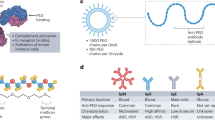
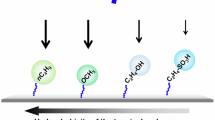
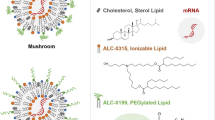
Messenger RNA lipid-nanoparticle-based therapies represent an emerging class of medicines for a variety of applications. However, anti-poly(ethylene glycol) (anti-PEG) antibodies generated by widely used PEGylated medicines and lipid nanoparticles hinder therapeutic efficacy upon repeated dosing. Here we report the chemical design, synthesis and optimization of high-density brush-shaped polymer lipids that reduce anti-PEG antibody binding to improve protein production consistency in repeated dosing. Brush-shaped polymer lipid parameters, including side chain length, degree of polymerization, anchor alkyl length and surface regimes on lipid nanoparticles modulate anti-PEG antibody binding affinity and control their blood circulation pharmacokinetics. Compared to widely used 1,2-dimyristoyl-rac-glycero-3-methoxypolyethylene glycol-2000, lipid nanoparticles containing brush-shaped polymer lipids generate superior therapeutic outcomes in protein replacement therapy and genome editing models, reformulating structure–activity guidelines for the design of PEG lipid substitutes. Overall, these findings contribute to the general effort in the development of lipid nanoparticles with low immunogenicity to overcome current roadblocks to nucleic acid medicines.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the data are available from the corresponding author upon request. Source data are provided with this paper.
Change history
04 June 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41563-025-02270-2
References
Rohner, E., Yang, R., Foo, K. S., Goedel, A. & Chien, K. R. Unlocking the promise of mRNA therapeutics. Nat. Biotechnol. 40, 1586–1600 (2022).
Zabaleta, N., Torella, L., Weber, N. D. & Gonzalez-Aseguinolaza, G. mRNA and gene editing: late breaking therapies in liver diseases. Hepatology 76, 869–887 (2022).
Cacicedo, M. L., Limeres, M. J. & Gehring, S. mRNA-based approaches to treating liver diseases. Cells 11, 3328 (2022).
Qin, S. et al. mRNA-based therapeutics: powerful and versatile tools to combat diseases. Signal Transduct. Target. Ther. 7, 166 (2022).
Xiao, Y. et al. Emerging mRNA technologies: delivery strategies and biomedical applications. Chem. Soc. Rev. 51, 3828–3845 (2022).
Hajj, K. A. & Whitehead, K. A. Tools for translation: non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2, 17056 (2017).
Mendell, J. R. et al. Current clinical applications of in vivo gene therapy with AAVs. Mol. Ther. 29, 464–488 (2021).
Milani, M. et al. Liver-directed lentiviral gene therapy corrects hemophilia A mice and achieves normal-range factor VIII activity in non-human primates. Nat. Commun. 13, 2454 (2022).
Nguyen, G. N. et al. A long-term study of AAV gene therapy in dogs with hemophilia A identifies clonal expansions of transduced liver cells. Nat. Biotechnol. 39, 47–55 (2021).
Hakim, C. H. et al. Cas9-specific immune responses compromise local and systemic AAV CRISPR therapy in multiple dystrophic canine models. Nat. Commun. 12, 6769 (2021).
Yang, Y. et al. A dual AAV system enables the Cas9-mediated correction of a metabolic liver disease in newborn mice. Nat. Biotechnol. 34, 334–338 (2016).
Villiger, L. et al. Treatment of a metabolic liver disease by in vivo genome base editing in adult mice. Nat. Med. 24, 1519–1525 (2018).
Schulz, M. et al. Binding and neutralizing anti-AAV antibodies: detection and implications for rAAV-mediated gene therapy. Mol. Ther. 31, 616–630 (2023).
Li, C. et al. Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. 19, 288–294 (2012).
Boutin, S. et al. Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum. Gene Ther. 21, 704–712 (2010).
Colella, P., Ronzitti, G. & Mingozzi, F. Emerging issues in AAV-mediated in vivo gene therapy. Mol. Ther. Methods Clin. Dev. 8, 87–104 (2018).
Dilliard, S. A. & Siegwart, D. J. Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nat. Rev. Mater. 8, 282–300 (2023).
Liu, S. et al. Membrane-destabilizing ionizable phospholipids for organ-selective mRNA delivery and CRISPR–Cas gene editing. Nat. Mater. 20, 701–710 (2021).
Wei, T. et al. Delivery of tissue-targeted scalpels: opportunities and challenges for in vivo CRISPR/Cas-based genome editing. ACS Nano 14, 9243–9262 (2020).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Ju, Y. et al. Anti-PEG antibodies boosted in humans by SARS-CoV-2 lipid nanoparticle mRNA vaccine. ACS Nano 16, 11769–11780 (2022).
Garofalo, C. et al. Different insight into amphiphilic PEG-PLA copolymers: influence of macromolecular architecture on the micelle formation and cellular uptake. Biomacromolecules 15, 403–415 (2014).
Zhou, K. et al. Hydrophobic domain structure of linear-dendritic poly(ethylene glycol) lipids affects RNA delivery of lipid nanoparticles. Mol. Pharm. 17, 1575–1585 (2020).
Dilliard, S. A., Cheng, Q. & Siegwart, D. J. On the mechanism of tissue-specific mRNA delivery by selective organ targeting nanoparticles. Proc. Natl Acad. Sci. USA 118, e2109256118 (2021).
Kenworthy, A. K., Hristova, K., Needham, D. & McIntosh, T. J. Range and magnitude of the steric pressure between bilayers containing phospholipids with covalently attached poly(ethylene glycol). Biophys. J. 68, 1921–1936 (1995).
Bai, L., Tan, L., Chen, L., Liu, S. & Wang, Y. Preparation and characterizations of poly(2-methyl-2-oxazoline) based antifouling coating by thermally induced immobilization. J. Mater. Chem. B 2, 7785–7794 (2014).
Allen, T. M., Hansen, C. B. & de Menezes, D. E. L. Pharmacokinetics of long-circulating liposomes. Adv. Drug Deliv. Rev. 16, 267–284 (1995).
Chen, S. et al. Influence of particle size on the in vivo potency of lipid nanoparticle formulations of sirna. J. Control. Release 235, 236–244 (2016).
Greenwald, R. B. PEG drugs: An overview. J. Control. Release 74, 159–171 (2001).
Gref, R. et al. Biodegradable long-circulating polymeric nanospheres. Science 263, 1600–1603 (1994).
Harris, J. M. & Chess, R. B. Effect of PEGylation on pharmaceuticals. Nat. Rev. Drug Discov. 2, 214–221 (2003).
Stavnsbjerg, C. et al. Accelerated blood clearance and hypersensitivity by PEGylated liposomes containing TLR agonists. J. Control. Release 342, 337–344 (2022).
Emam, S. E. et al. Anti-PEG IgM production and accelerated blood clearance phenomenon after the administration of PEGylated exosomes in mice. J. Control. Release 334, 327–334 (2021).
Kang, D. D. et al. Engineering LNPs with polysarcosine lipids for mRNA delivery. Bioact. Mater. 37, 86–93 (2024).
Da Silva Sanchez, A. J. et al. Substituting poly(ethylene glycol) lipids with poly(2-ethyl-2-oxazoline) lipids improves lipid nanoparticle repeat dosing. Adv. Healthc. Mater. 13, 2304033 (2024).
Chen, J., Rizvi, A., Patterson, J. P. & Hawker, C. J. Discrete libraries of amphiphilic poly(ethylene glycol) graft copolymers: synthesis, assembly, and bioactivity. J. Am. Chem. Soc. 144, 19466–19474 (2022).
Sun, Y. et al. In vivo editing of lung stem cells for durable gene correction in mice. Science 384, 1196–1202 (2024).
Lian, X. et al. Bone-marrow-homing lipid nanoparticles for genome editing in diseased and malignant haematopoietic stem cells. Nat. Nanotechnol. 19, 1409–1417 (2024).
Wang, X. et al. Preparation of selective organ-targeting (SORT) lipid nanoparticles (LNPs) using multiple technical methods for tissue-specific mRNA delivery. Nat. Protoc. 18, 265–291 (2023).
Acknowledgements
The research was supported by the Welch Foundation (I-2123-20220031); the National Institutes of Health (NIH) National Institute of Biomedical Imaging and Bioengineering (NIBIB; R01 5R01EB025192-06) and National Cancer Institute (NCI; R01 CA269787-01); and the Cystic Fibrosis Foundation (CFF; SIEGWA18XX0 and SIEGWA21XX0). J.G.M. was supported in part by NIH UL1TR003163, NIH 1P30DK127984-01A1 and NIH 1P01HL160487-01. C.L. was supported by NIH; R01GM143723. We acknowledge support from H. Zhu and his lab for assistance with the FAH knock-out mouse study, and from the University of Texas Southwestern Small Animal Imaging Resource (NCI P30CA142543), Whole Brain Microscopy Facility (SCR_017949), Metabolic Phenotyping Core, Tissue Management Shared Resource (P30CA142543) and Histo Pathology Core. LNPs and mice in Figs 1a,b, 3b,i, 4a,e, 5a and 6a,f created with BioRender.com.
Author information
Authors and Affiliations
Contributions
Y.X. and D.J.S. conceived and designed the experiments and wrote the manuscript. Y.X., X.L., Y.S., Y.-C.S., A.V., Z.C., A.G., J.G.M., S.C., L.Z., E.G., X.W., L.F., Y.Y., M.I.D. and C.L. performed experiments. All authors discussed the results and commented on the manuscript. D.J.S. directed the research.
Corresponding author
Ethics declarations
Competing interests
The University of Texas Southwestern has filed patent applications on the technologies described in this manuscript with some co-authors listed as inventors. D.J.S. discloses financial interests in ReCode Therapeutics, Signify Bio, Jumble Therapeutics and Tome Biosciences.
Peer review
Peer review information
Nature Materials thanks Camilla Foged, Liangfang Zhang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–54, Tables 1–7, Materials and Methods, Results and Discussion and references.
Supplementary Data 1
Source data for Supplementary Figs. 1, 3–12, 14, 15, 19 and 21–23.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 6d
Unprocessed western blots.
Source Data Fig. 6i
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xiao, Y., Lian, X., Sun, Y. et al. High-density brush-shaped polymer lipids reduce anti-PEG antibody binding for repeated administration of mRNA therapeutics. Nat. Mater. 24, 1840–1851 (2025). https://doi.org/10.1038/s41563-024-02116-3
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41563-024-02116-3
This article is cited by
-
mRNA technology for the prevention and treatment of HIV-1 infection
Nature Reviews Bioengineering (2026)
-
On the horizon in biomedical engineering
Nature Biomedical Engineering (2026)
-
Design principles of lipid nanoparticles for RNA delivery
Nature Reviews Bioengineering (2026)
-
Emerging nanomedicine for liver diseases treatment
Journal of Nanobiotechnology (2025)
-
Making way for PEG alternatives
Nature Materials (2025)