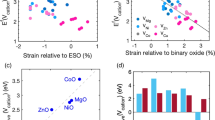
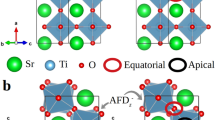
Abstract
Oxygen vacancies in oxide materials, although demonstrated to be beneficial for many applications, are hard to be generated and manipulated as desired, particularly for bulk materials with a large size and limited surface area. Here, by simply coupling the thermal activation with a simultaneously applied electric field, we efficiently generate ordered oxygen vacancies within bulk crystals of ternary SrAl2O4, binary TiO2 and other common oxide materials, which give rise to superior functionalities. We expect that this approach offers a general and practical way for the vacancy engineering of oxide materials and holds great promise for their applications.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data and relevant information are available within the Article and its Supplementary Information. Data supporting the findings of this study are available from the corresponding authors upon reasonable request.
References
Tuller, H. L. & Bishop, S. R. Point defects in oxides: tailoring materials through defect engineering. Annu. Rev. Mater. Res. 41, 369–398 (2011).
Mundet, B. et al. Local strain-driven migration of oxygen vacancies to apical sites in YBa2Cu3O7–x. Nanoscale 12, 5922–5931 (2020).
Goodenough, J. B. Ceramic technology—oxide-ion conductors by design. Nature 404, 821–823 (2000).
Kim, H.-S. et al. Oxygen vacancies enhance pseudocapacitive charge storage properties of MoO3–x. Nat. Mater. 16, 454–460 (2017).
Chen, X., Liu, L., Yu, P. Y. & Mao, S. S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science 331, 746–750 (2011).
Lv, Y. et al. Production of visible activity and UV performance enhancement of ZnO photocatalyst via vacuum deoxidation. Appl. Catal. B 138, 26–32 (2013).
Chen, X., Liu, L. & Huang, F. Black titanium dioxide (TiO2) nanomaterials. Chem. Soc. Rev. 44, 1861–1885 (2015).
Wang, G., Yang, Y., Han, D. & Li, Y. Oxygen defective metal oxides for energy conversion and storage. Nano Today 13, 23–39 (2017).
Wang, J., Chen, R., Xiang, L. & Komarneni, S. Synthesis, properties and applications of ZnO nanomaterials with oxygen vacancies: a review. Ceram. Int. 44, 7357–7377 (2018).
Yuan, Y. et al. Ordering heterogeneity of MnO6 octahedra in tunnel-structured MnO2 and its influence on ion storage. Joule 3, 471–484 (2019).
Blanc, J. & Staebler, D. L. Electrocoloration in SrTiO3: vacancy drift and oxidation-reduction of transition metals. Phys. Rev. B 4, 3548–3557 (1971).
Ueno, K. et al. Electric-field-induced superconductivity in an insulator. Nat. Mater. 7, 855–858 (2008).
Jeong, J. et al. Suppression of metal-insulator transition in VO2 by electric field-induced oxygen vacancy formation. Science 339, 1402–1405 (2013).
Nian, Y. B., Strozier, J., Wu, N. J., Chen, X. & Ignatiev, A. Evidence for an oxygen diffusion model for the electric pulse induced resistance change effect in transition-metal oxides. Phys. Rev. Lett. 98, 146403 (2007).
Li, J. et al. Orientational alignment of oxygen vacancies: electric-field- inducing conductive channels in TiO2 film to boost photocatalytic conversion of CO2 into CO. Nano Lett. 21, 5060–5067 (2021).
Matsuzawa, T., Aoki, Y., Takeuchi, N. & Murayama, Y. New long phosphorescent phosphor with high brightness, SrAl2O4:Eu2+,Dy3+. J. Electrochem. Soc. 143, 2670–2673 (1996).
Lazic, I., Bosch, E. G. T. & Lazar, S. Phase contrast STEM for thin samples: integrated differential phase contrast. Ultramicroscopy 160, 265–280 (2016).
Rose, H. Nonstandard imaging methods in electron-microscopy. Ultramicroscopy 2, 251–267 (1977).
Zhang, Q. H. et al. Atomic-resolution imaging of electrically induced oxygen vacancy migration and phase transformation in SrCoO2.5–σ. Nat. Commun. 8, 6 (2017).
Maier, J. Defect chemistry: composition, transport, and recations in the solid state; Part I: thermodynamics. Angew. Chem. Int. Ed. Engl. 32, 313–335 (1993).
Lu, Q. Y. et al. Electrochemically triggered metal-insulator transition between VO2 and V2O5. Adv. Funct. Mater. 28, 1803024 (2018).
Mueller, D. N., Machala, M. L., Bluhm, H. & Chueh, W. C. Redox activity of surface oxygen anions in oxygen-deficient perovskite oxides during electrochemical reactions. Nat. Commun. 6, 6097 (2015).
Onda, K., Li, B. & Petek, H. Two-photon photoemission spectroscopy of TiO2(110) surfaces modified by defects and O2 or H2O adsorbates. Phys. Rev. B 70, 045415 (2004).
Szot, K., Bihlmayer, G. & Speier, W. Nature of the resistive switching phenomena in TiO2 and SrTiO3: origin of the reversible insulator–metal transition. Solid State Phys. 65, 353–559 (2014).
Szot, K., Speier, W., Bihlmayer, G. & Waser, R. Switching the electrical resistance of individual dislocations in single-crystalline SrTiO3. Nat. Mater. 5, 312–320 (2006).
Joll, K., Schienbein, P., Rosso, K. M. & Blumberger, J. J. Machine learning the electric field response of condensed phase systems using perturbed neural network potentials. Nat. Commun. 15, 8192 (2024).
Shah, A. A., Dar, A. B. & Shrivastava, M. Revisiting the origin of non-volatile resistive switching in MoS2 atomristor. npj 2D Mater. Appl. 8, 80 (2024).
Rinkevicius, Z. et al. A polarizable coarse-grained model for metal, metal oxide and composite metal/metal oxide nanoparticles and its applications. Phys. Chem. Chem. Phys. 24, 27742–27750 (2022).
Nandi, S. K., Liu, X., Venkatachalam, D. K. & Elliman, R. G. Effect of electrode roughness on electroforming in HfO2 and defect-induced moderation of electric-field enhancement. Phys. Rev. Appl. 4, 064010 (2015).
Zhai, B. & Huang, Y. Green afterglow of undoped SrAl2O4. Nanomaterials 11, 2331 (2021).
Canaza-Mamani, E. A. et al. TL and EPR characteristics of SrAl2O4 phosphor prepared by solid‐state reaction method. J. Lumin. 245, 118585 (2022).
Hoang, K. Defects and persistent luminescence in Eu-doped SrAl2O4. Phys. Rev. Appl. 19, 024060 (2023).
Van den Eeckhout, K., Bos, A. J. J., Poelman, D. & Smet, P. F. Revealing trap depth distributions in persistent phosphors. Phys. Rev. B 87, 045126 (2013).
Qu, B., Zhang, B., Wang, L., Zhou, R. & Zeng, X. C. Mechanistic study of the persistent luminescence of CaAl2O4:Eu,Nd. Chem. Mater. 27, 2195–2202 (2015).
De Clercq, O. Q., Du, J., Smet, P. F., Joos, J. J. & Poelman, D. Predicting the afterglow duration in persistent phosphors: a validated approach to derive trap depth distributions. Phys. Chem. Chem. Phys. 20, 30455–30465 (2018).
Van den Eeckhout, K., Smet, P. F. & Poelman, D. Persistent luminescence in Eu2+-doped compounds: a review. Materials 3, 2536–2566 (2010).
Zeng, P., Wei, X., Yin, M. & Chen, Y. Investigation of the long afterglow mechanism in SrAl2O4:Eu2+/Dy3+ by optically stimulated luminescence and thermoluminescence. J. Lumin. 199, 400–406 (2018).
Clabau, F. et al. Formulation of phosphorescence mechanisms in inorganic solids based on a new model of defect conglomeration. Chem. Mater. 18, 3212–3220 (2006).
Clabau, F. et al. Mechanism of phosphorescence appropriate for the long-lasting phosphors Eu2+-doped SrAl2O4 with codopants Dy3+ and B3+. Chem. Mater. 17, 3904–3912 (2005).
Gloter, A., Ewels, C., Umek, P., Arcon, D. & Colliex, C. Electronic structure of titania-based nanotubes investigated by EELS spectroscopy. Phys. Rev. B 80, 035413 (2009).
Stoyanov, E., Langenhorst, F. & Steinle-Neumann, G. The effect of valence state and site geometry on Ti L3,2 and O K electron energy-loss spectra of TixOy phases. Am. Mineral. 92, 577–586 (2007).
Su, T. et al. An insight into the role of oxygen vacancy in hydrogenated TiO2 nanocrystals in the performance of dye-sensitized solar cells. ACS Appl. Mater. Interfaces 7, 3754–3763 (2015).
Zou, X. et al. Facile synthesis of thermal- and photostable titania with paramagnetic oxygen vacancies for visible-light photocatalysis. Chem. Eur. J. 19, 2866–2873 (2013).
Kang, Q. et al. Reduced TiO2 nanotube arrays for photoelectrochemical water splitting. J. Mater. Chem. A 1, 5766–5774 (2013).
Han, G., Kim, J. Y., Kim, K.-J., Lee, H. & Kim, Y.-M. Controlling surface oxygen vacancies in Fe-doped TiO2 anatase nanoparticles for superior photocatalytic activities. Appl. Surf. Sci. 507, 144916 (2020).
Thomas, A. G. et al. Comparison of the electronic structure of anatase and rutile TiO2 single-crystal surfaces using resonant photoemission and x-ray absorption spectroscopy. Phys. Rev. B 75, 035105 (2007).
Shin, J.-Y., Joo, J. H., Samuelis, D. & Maier, J. Oxygen-deficient TiO2–δ nanoparticles via hydrogen reduction for high rate capability lithium batteries. Chem. Mater. 24, 543–551 (2012).
Li, H.-Y. et al. Electrochemically grown nanocrystalline V2O5 as high-performance cathode for sodium-ion batteries. J. Power Sources 285, 418–424 (2015).
Kresse, G. & Furthmuller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 6, 15–50 (1996).
Kresse, G. Ab-initio molecular-dynamics for liquid-metals. J. Non-Cryst. Solids 193, 222–229 (1995).
Kresse, G. & Hafner, J. Ab-initio molecular-dynamics for open-shell transition-metals. Phys. Rev. B 48, 13115–13118 (1993).
Blochl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Nunes, R. W. & Gonze, X. Berry-phase treatment of the homogeneous electric field perturbation in insulators. Phys. Rev. B 63, 155107 (2001).
Souza, I., Iniguez, J. & Vanderbilt, D. First-principles approach to insulators in finite electric fields. Phys. Rev. Lett. 89, 117602 (2002).
Henkelman, G. & Jonsson, H. Improved tangent estimate in the nudged elastic band method for finding minimum energy paths and saddle points. J. Chem. Phys. 113, 9978–9985 (2000).
Henkelman, G., Uberuaga, B. P. & Jonsson, H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 113, 9901–9904 (2000).
Larson, A. C. & Von Dreele, R. B. General structure analysis system (GSAS). Los Alamos National Laboratory Report LAUR 86–748 (1994).
Acknowledgements
We acknowledge C. Nan, L. Gu, S. Du, Z. Lin and L. Li for many discussions and helpful suggestions. R.S.-Y. acknowledges financial support from NSF DMR-1809439 for the TiO2 STEM characterizations. Use of the Advanced Photon Source (APS) 9-BM beamline at Argonne National Laboratory, Office of Science User Facility, was supported by the US Department of Energy, Office of Science, Office of Basic Energy Sciences, under contract no. DE-AC02-06CH11357.
Author information
Authors and Affiliations
Contributions
K.C. conceived the idea, designed the experiments and guided the whole project. X.Y., Z.C. and G.L. synthesized the afterglow materials and performed the corresponding measurements. Z.T. and M.Z. synthesized the binary oxide samples with O defects and performed the corresponding measurements. Q.Z. carried out the electron microscopy characterizations for the SrAl2O4 sample. Y.Y. and R.S.-Y. carried out the electron microscopy characterizations for the TiO2 sample. Y.Z. and X.J. performed the first-principles calculations. T.W. and Y.Y. conducted the synchrotron analyses. All authors contributed to data interpretation and discussed the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–27 and Tables 1 and 2.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, K., Yuan, X., Tian, Z. et al. A facile approach for generating ordered oxygen vacancies in metal oxides. Nat. Mater. 24, 835–842 (2025). https://doi.org/10.1038/s41563-025-02171-4
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41563-025-02171-4
This article is cited by
-
Low-temperature conformal SnO2 coating enables efficient printable mesoscopic perovskite solar cells with industrial scalability
PhotoniX (2026)
-
Multisensory Neuromorphic Devices: From Physics to Integration
Nano-Micro Letters (2026)
-
Defect-engineered black tetragonal-tungsten-bronze ferroelectric crystal for full spectrum absorption and broadband photoelectronic conversion
Nature Communications (2025)