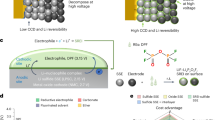
Abstract
Dealloying reactions underpin the operation of next-generation battery electrodes and are also a synthesis route for porous metals, but the influence of mechanical stress on these processes is not well understood. Here we investigate how the applied stack pressure affects structural evolution and electrochemical reversibility during the alloying/dealloying of Li alloy materials (Li–Al, Li–Sn, Li–In and Li–Si) using solid-state and liquid electrolytes. The extent of porosity formation during the dealloying of metals is found to be universally governed by stack pressure, with pressures of at least 20% of the yield strength required to achieve ~80% relative density. This concept is correlated to the cycling of alloy electrodes in solid-state batteries, with a yield-strength-dependent threshold pressure needed for reversible high Li-storage capacity due to densification. With this understanding, we design Al and Si anodes with a densified interfacial layer enabling stable cycling at low stack pressures (2 MPa), providing guidance towards practical high-energy solid-state batteries.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
McCue, I., Benn, E., Gaskey, B. & Erlebacher, J. Dealloying and dealloyed materials. Ann. Rev. Mater. Res. 46, 263–286 (2016).
Weissmüller, J., Newman, R. C., Jin, H.-J., Hodge, A. M. & Kysar, J. W. Nanoporous metals by alloy corrosion: formation and mechanical properties. MRS Bull. 34, 577–586 (2009).
Wittstock, G. et al. Nanoporous gold: from structure evolution to functional properties in catalysis and electrochemistry. Chem. Rev. 123, 6716–6792 (2023).
Erlebacher, J., Aziz, M. J., Karma, A., Dimitrov, N. & Sieradzki, K. Evolution of nanoporosity in dealloying. Nature 410, 450–453 (2001).
Chen, Q. & Sieradzki, K. Mechanisms and morphology evolution in dealloying. J. Electrochem. Soc. 160, C226–C231 (2013).
Weissmueller, J. & Sieradzki, K. Dealloyed nanoporous materials with interface-controlled behavior. MRS Bull. 43, 14–19 (2018).
Chen, Q. & Sieradzki, K. Spontaneous evolution of bicontinuous nanostructures in dealloyed Li-based systems. Nat. Mater. 12, 1102–1106 (2013).
McDowell, M. T. et al. In situ TEM of two-phase lithiation of amorphous silicon nanospheres. Nano Lett. 13, 758–764 (2013).
Obrovac, M. N. & Chevrier, V. L. Alloy negative electrodes for Li-ion batteries. Chem. Rev. 114, 11444–11502 (2014).
McDowell, M. T., Lee, S. W., Nix, W. D. & Cui, Y. 25th anniversary article: understanding the lithiation of silicon and other alloying anodes for lithium-ion batteries. Adv. Mater. 25, 4966–4985 (2013).
Lewis, J. A., Cavallaro, K. A., Liu, Y. & McDowell, M. T. The promise of alloy anodes for solid-state batteries. Joule 6, 1418–1430 (2022).
Liu, Y. et al. In situ transmission electron microscopy observation of pulverization of aluminum nanowires and evolution of the thin surface Al2O3 layers during lithiation–delithiation cycles. Nano Lett. 11, 4188–4194 (2011).
Heligman, B. T., Scanlan, K. P. & Manthiram, A. An in-depth analysis of the transformation of tin foil anodes during electrochemical cycling in lithium-ion batteries. J. Electrochem. Soc. 168, 120544 (2021).
Geng, K. & Sieradzki, K. Dealloying at high homologous temperature: morphology diagrams. J. Electrochem. Soc. 164, C330–C337 (2017).
Huber, N., Viswanath, R. N., Mameka, N., Markmann, J. & Weißmüller, J. Scaling laws of nanoporous metals under uniaxial compression. Acta Mater. 67, 252–265 (2014).
Jin, H.-J. et al. Deforming nanoporous metal: role of lattice coherency. Acta Mater. 57, 2665–2672 (2009).
Jin, H.-J., Weissmueller, J. & Farkas, D. Mechanical response of nanoporous metals: a story of size, surface stress, and severed struts. MRS Bull. 43, 35–42 (2018).
Parida, S. et al. Volume change during the formation of nanoporous gold by dealloying. Phys. Rev. Lett. 97, 035504 (2006).
Hirai, T., Yoshimatsu, I. & Yamaki, J. Influence of electrolyte on lithium cycling efficiency with pressurized electrode stack. J. Electrochem. Soc. 141, 611–614 (1994).
Yin, X. et al. Insights into morphological evolution and cycling behaviour of lithium metal anode under mechanical pressure. Nano Energy 50, 659–664 (2018).
Zhang, X. et al. Rethinking how external pressure can suppress dendrites in lithium metal batteries. J. Electrochem. Soc. 166, A3639–A3652 (2019).
Fang, C. et al. Pressure-tailored lithium deposition and dissolution in lithium metal batteries. Nat. Energy 6, 987–994 (2021).
Albertus, P. et al. Challenges for and pathways toward Li-metal-based all-solid-state batteries. ACS Energy Lett. 6, 1399–1404 (2021).
Tan, D. H. S., Meng, Y. S. & Jang, J. Scaling up high-energy-density sulfidic solid-state batteries: a lab-to-pilot perspective. Joule 6, 1755–1769 (2022).
Wang, H. et al. The progress on aluminum-based anode materials for lithium-ion batteries. J. Mater. Chem. A 8, 25649–25662 (2020).
Zheng, T., Kramer, D., Mönig, R. & Boles, S. T. Aluminum foil anodes for Li-ion rechargeable batteries: the role of Li solubility within β-LiAl. ACS Sustain. Chem. Eng. 10, 3203–3210 (2022).
Webb, S. A., Baggetto, L., Bridges, C. A. & Veith, G. M. The electrochemical reactions of pure indium with Li and Na: anomalous electrolyte decomposition, benefits of FEC additive, phase transitions and electrode performance. J. Power Sources 248, 1105–1117 (2014).
Heligman, B. T. & Manthiram, A. Elemental foil anodes for lithium-ion batteries. ACS Energy Lett. 6, 2666–2672 (2021).
Chen, T. et al. Benchmarking the degradation behavior of aluminum foil anodes for lithium-ion batteries. Batter. Supercaps 6, e202200363 (2023).
Sakka, Y. et al. Investigating plastic deformation between silicon and solid electrolyte in all-solid-state batteries using operando X-ray tomography. J. Electrochem. Soc. 171, 070536 (2024).
Wu, X. et al. Operando visualization of morphological dynamics in all‐solid‐state batteries. Adv. Energy Mater. 9, 1901547 (2019).
Obrovac, M. N., Christensen, L., Le, D. B. & Dahn, J. R. Alloy design for lithium-ion battery anodes. J. Electrochem. Soc. 154, A849 (2007).
Tarczon, J., Halperin, W., Chen, S. & Brittain, J. Vacancy-antistructure defect interaction diffusion in β-LiAl and β-LiIn. Mater. Sci. Eng. A 101, 99–108 (1988).
Jeong, W. J. et al. Electrochemical behavior of elemental alloy anodes in solid-state batteries. ACS Energy Lett. 9, 2554–2563 (2024).
McCue, I., Karma, A. & Erlebacher, J. Pattern formation during electrochemical and liquid metal dealloying. MRS Bull. 43, 27–34 (2018).
Wang, C., Zhu, G., Liu, P. & Chen, Q. Monolithic nanoporous Zn anode for rechargeable alkaline batteries. ACS Nano 14, 2404–2411 (2020).
Nairn, J. A. Numerical simulations of transverse compression and densification in wood. Wood Fiber Sci. 4, 576–591 (2006).
Gibson, L. J. & Ashby, M. F. Cellular Solids: Structure and Properties (Cambridge Univ. Press, 1997).
Hodge, A. M. et al. Scaling equation for yield strength of nanoporous open-cell foams. Acta Mater. 55, 1343–1349 (2007).
Liu, L.-Z., Ye, X.-L. & Jin, H.-J. Interpreting anomalous low-strength and low-stiffness of nanoporous gold: quantification of network connectivity. Acta Mater. 118, 77–87 (2016).
Tan, D. H. S. et al. Carbon-free high-loading silicon anodes enabled by sulfide solid electrolytes. Science 373, 1494–1499 (2021).
Berla, L. A., Lee, S. W., Cui, Y. & Nix, W. D. Mechanical behavior of electrochemically lithiated silicon. J. Power Sources 273, 41–51 (2015).
Liu, Y. et al. Aluminum foil negative electrodes with multiphase microstructure for all-solid-state Li-ion batteries. Nat. Commun. 14, 3975 (2023).
Han, S. Y. et al. Stress evolution during cycling of alloy-anode solid-state batteries. Joule 5, 2450–2465 (2021).
Cangaz, S. et al. Enabling high‐energy solid‐state batteries with stable anode interphase by the use of columnar silicon anodes. Adv. Energy Mater. 10, 2001320 (2020).
Cao, D. et al. Long‐cycling sulfide‐based all‐solid‐state batteries enabled by electrochemo‐mechanically stable electrodes. Adv. Mater. 34, 2200401 (2022).
Lee, J. et al. Dry pre‐lithiation for graphite‐silicon diffusion‐dependent electrode for all‐solid‐state battery. Adv. Energy Mater. 13, 2300172 (2023).
Huo, H. et al. Chemo-mechanical failure mechanisms of the silicon anode in solid-state batteries. Nat. Mater. 23, 543–551 (2024).
Yamamoto, M., Terauchi, Y., Sakuda, A., Kato, A. & Takahashi, M. Effects of volume variations under different compressive pressures on the performance and microstructure of all-solid-state batteries. J. Power Sources 473, 228595 (2020).
Kim, J. Y. et al. Graphite–silicon diffusion‐dependent electrode with short effective diffusion length for high‐performance all‐solid‐state batteries. Adv. Energy Mater. 12, 2103108 (2022).
Xu, X. et al. Nano silicon anode without electrolyte adding for sulfide‐based all‐solid‐state lithium‐ion batteries. Small 19, 2302934 (2023).
Fan, Z. et al. In-situ prelithiation of electrolyte-free silicon anode for sulfide all-solid-state batteries. eTransportation 18, 100277 (2023).
Cao, D. et al. Unveiling the mechanical and electrochemical evolution of nanosilicon composite anodes in sulfide‐based all‐solid‐state batteries. Adv. Energy Mater. 13, 2203969 (2023).
Zhou, L. et al. Li3–xZrx(Ho/Lu)1–xCl6 solid electrolytes enable ultrahigh-loading solid-state batteries with a prelithiated Si anode. ACS Energy Lett. 8, 3102–3111 (2023).
Luo, S. et al. Growth of lithium-indium dendrites in all-solid-state lithium-based batteries with sulfide electrolytes. Nat. Commun. 12, 6968 (2021).
Huang, Y., Shao, B. & Han, F. Li alloy anodes for high-rate and high-areal-capacity solid-state batteries. J. Mater. Chem. A 10, 12350–12358 (2022).
Fan, Z. et al. Long‐cycling all‐solid‐state batteries achieved by 2D interface between prelithiated aluminum foil anode and sulfide electrolyte. Small 18, 2204037 (2022).
Pan, H. et al. Carbon-free and binder-free Li–Al alloy anode enabling an all-solid-state Li-S battery with high energy and stability. Sci. Adv. 8, eabn4372 (2022).
Zhang, W. et al. Interfacial processes and influence of composite cathode microstructure controlling the performance of all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 9, 17835–17845 (2017).
Oliver, W. C. & Pharr, G. M. An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J. Mater. Res. 7, 1564–1583 (1992).
Acknowledgements
We acknowledge support from the National Science Foundation, award no. DMR-2209202 (M.T.M.). Partial support is acknowledged from Novelis Inc. This work was performed in part at the Georgia Tech Institute for Matter and Systems, a member of the National Nanotechnology Coordinated Infrastructure (NNCI), which is supported by the National Science Foundation (ECCS-2025462).
Author information
Authors and Affiliations
Contributions
Conceptualization: C.W. and M.T.M. Methodology: C.W. Investigation: C.W., Y.L., W.J.J., T.C., M.L., D.L.N., E.P.A., S.G.Y. and K.A.C. Formal analysis: C.W., M.L., S.X. and M.T.M. Validation: C.W. and M.T.M. Writing—original draft: C.W. Writing—review and editing: C.W., S.D., D.M., R.G., S.X. and M.T.M. Visualization: C.W. and M.T.M. Project administration: S.D., D.M., R.G. and M.T.M. Resources: M.T.M. Supervision: M.T.M. Funding acquisition: M.T.M.
Corresponding author
Ethics declarations
Competing interests
C.W., Y.L., T.C., S.D., D.M., R.G. and M.T.M. are inventors on patent applications related to alloy anode materials for Li batteries. The other authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Yuki Orikasa, Haoshen Zhou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Pressure effects on Li trapping behaviour during electrochemical dealloying of metals with liquid and solid-state electrolytes.
Trapped Li capacity versus applied stack pressure \(\sigma\) during electrochemical dealloying of the three metals in liquid electrolyte (a) and solid-state electrolyte (b). The trapped Li capacity was measured from the Li dealloying current curves in Fig. 2a–c and normalized to the lithiation capacity measured from the Li alloying curves in Fig. 1a–c.
Extended Data Fig. 2 The influence of the dealloying rate using solid-state electrolyte on morphology evolution.
a, XRD characterization of In electrodes after galvanostatic dealloying at different current densities using the same stack pressure (1 MPa). b–d, Cross-sectional SEM images of In electrodes after galvanostatic dealloying at different current densities under 1 MPa of stack pressure. All scale bars are 1 µm.
Extended Data Fig. 3 In situ stack pressure measurement of a cell with a Si wafer electrode during alloying and dealloying using SSE at 2 MPa stack pressure.
a, Galvanostatic voltage curve from the alloying of a Si wafer working electrode along with measured stress in the cell. A current density of 0.1 mA cm−2 was used. b, Current density curve from potentiostatic dealloying (1.0 V versus Li/Li+) of a Si wafer electrode along with measured stress in the cell. A LiIn counter electrode was used in both cases.
Extended Data Fig. 4 Pressure effects on relative density and Li trapping behaviour during electrochemical dealloying of Si with liquid and solid-state electrolytes.
a, Measured relative density \(\varphi\) versus applied stack pressure \(\sigma\) during electrochemical dealloying of Si. Relative density and error bars were calculated based on measurements at 10 different positions. Data in (a) are presented as mean values ± the standard error of the mean, where the number of replicates (n) is 10. b, Trapped Li capacity versus applied stack pressure \(\sigma\) during electrochemical dealloying of Si. The trapped Li capacity was measured from the Li dealloying current curves in Fig. 4d and normalized to the lithiation capacity measured from the Li alloying curves in Fig. 4a.
Extended Data Fig. 5 EDS maps of the Al and Si anodes with In coating.
a, b, Al. c, d, Si. All scale bars are 5 µm.
Supplementary information
Supplementary Information
Supplementary Figs. 1–19, Tables 1–4, Equations (1) and (2) and References.
Source data
Source Data Fig. 1
Source data for the electrochemical tests shown in Fig. 1, including dealloying of Li from various metal electrodes in liquid electrolytes and SSEs.
Source Data Fig. 2
Source data for the electrochemical tests shown in Fig. 2, including dealloying of Li from various metal electrolytes in liquid electrolytes and SSEs.
Source Data Fig. 4
Source data for the electrochemical tests shown in Fig. 4, including the electrochemical lithiation and dealloying data for silicon electrodes.
Source Data Fig. 5
Source data for the electrochemical tests shown in Fig. 5, including the galvanostatic charge–discharge and cycling data of Al, Sn, In and Si electrodes in SSBs at different stack pressures.
Source Data Fig. 6
Source data for the electrochemical tests shown in Fig. 6, including the electrochemical cycling behaviour of Al and Si electrodes with In coatings.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, C., Liu, Y., Jeong, W.J. et al. The influence of pressure on lithium dealloying in solid-state and liquid electrolyte batteries. Nat. Mater. 24, 907–916 (2025). https://doi.org/10.1038/s41563-025-02198-7
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41563-025-02198-7