Abstract
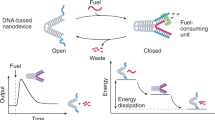
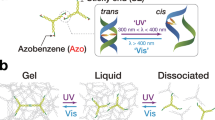
As active matter, cells exhibit non-equilibrium structures and behaviours such as reconfiguration, motility and division. These capabilities arise from the collective action of biomolecular machines continuously converting photoenergy or chemical energy into mechanical energy. Constructing similar dynamic processes in vitro presents opportunities for developing life-like intelligent soft materials. Here we report an active fluid formed from the liquid–liquid phase separation of photoresponsive DNA nanomachines. The photofluids can orchestrate and amplify nanoscale mechanical movements by orders of magnitude to produce macroscopic cell-like behaviours including elongation, division and rotation. We identify two dissipative processes in the DNA droplets, photoalignment and photofibrillation, which are crucial for harnessing stochastic molecular motions cooperatively. Our results demonstrate an active liquid molecular system that consumes photoenergy to create ordered out-of-equilibrium structures and behaviours. This system may help elucidate the physical principles underlying cooperative motion in active matter and pave the way for developing programmable interactive materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated during this study are included in the Article and its Supplementary Information. Source data are provided with this paper.
References
SenGupta, S., Parent, C. A. & Bear, J. E. The principles of directed cell migration. Nat. Rev. Mol. Cell Biol. 22, 529–547 (2021).
Dattler, D. et al. Design of collective motions from synthetic molecular switches, rotors, and motors. Chem. Rev. 120, 310–433 (2020).
Moulin, E., Faour, L., Carmona-Vargas, C. C. & Giuseppone, N. From molecular machines to stimuli-responsive materials. Adv. Mater. 32, 1906036 (2020).
Xu, F. & Feringa, B. L. Photoresponsive supramolecular polymers: from light-controlled small molecules to smart materials. Adv. Mater. 35, 2204413 (2023).
Pezzato, C., Cheng, C., Stoddart, J. F. & Astumian, R. D. Mastering the non-equilibrium assembly and operation of molecular machines. Chem. Soc. Rev. 46, 5491–5507 (2017).
Popkin, G. The physics of life. Nature 529, 16–18 (2016).
Lancia, F., Ryabchun, A. & Katsonis, N. Life-like motion driven by artificial molecular machines. Nat. Rev. Chem. 3, 536–551 (2019).
Köhler, S., Schaller, V. & Bausch, A. R. Structure formation in active networks. Nat. Mater. 10, 462–468 (2011).
Mizuno, D., Tardin, C., Schmidt, C. F. & MacKintosh, F. C. Nonequilibrium mechanics of active cytoskeletal networks. Science 315, 370–373 (2007).
Ndlec, F. J., Surrey, T., Maggs, A. C. & Leibler, S. Self-organization of microtubules and motors. Nature 389, 305–308 (1997).
Sanchez, T., Chen, D. T. N., DeCamp, S. J., Heymann, M. & Dogic, Z. Spontaneous motion in hierarchically assembled active matter. Nature 491, 431–434 (2012).
Adkins, R. et al. Dynamics of active liquid interfaces. Science 377, 768–772 (2022).
Wu, K.-T. et al. Transition from turbulent to coherent flows in confined three-dimensional active fluids. Science 355, eaal1979 (2017).
Tayar, A. M. et al. Controlling liquid–liquid phase behaviour with an active fluid. Nat. Mater. 22, 1401–1408 (2023).
Keya, J. J. et al. DNA-assisted swarm control in a biomolecular motor system. Nat. Commun. 9, 453 (2018).
Keber, F. C. et al. Topology and dynamics of active nematic vesicles. Science 345, 1135–1139 (2014).
Ross, T. D. et al. Controlling organization and forces in active matter through optically defined boundaries. Nature 572, 224–229 (2019).
Sumino, Y. et al. Large-scale vortex lattice emerging from collectively moving microtubules. Nature 483, 448–452 (2012).
Nitta, T., Wang, Y., Du, Z., Morishima, K. & Hiratsuka, Y. A printable active network actuator built from an engineered biomolecular motor. Nat. Mater. 20, 1149–1155 (2021).
Wollman, A. J. M., Sanchez-Cano, C., Carstairs, H. M. J., Cross, R. A. & Turberfield, A. J. Transport and self-organization across different length scales powered by motor proteins and programmed by DNA. Nat. Nanotechnol. 9, 44–47 (2014).
Kinbara, K. & Aida, T. Toward intelligent molecular machines: directed motions of biological and artificial molecules and assemblies. Chem. Rev. 105, 1377–1400 (2005).
Baroncini, M., Silvi, S. & Credi, A. Photo- and redox-driven artificial molecular motors. Chem. Rev. 120, 200–268 (2020).
Kassem, S. et al. Artificial molecular motors. Chem. Soc. Rev. 46, 2592–2621 (2017).
van Delden, R. A., Koumura, N., Harada, N. & Feringa, B. L. Unidirectional rotary motion in a liquid crystalline environment: color tuning by a molecular motor. Proc. Natl Acad. Sci. USA 99, 4945–4949 (2002).
Orlova, T. et al. Revolving supramolecular chiral structures powered by light in nanomotor-doped liquid crystals. Nat. Nanotechnol. 13, 304–308 (2018).
Hou, J. et al. Phototriggered complex motion by programmable construction of light-driven molecular motors in liquid crystal networks. J. Am. Chem. Soc. 144, 6851–6860 (2022).
Lan, R. et al. Amplifying molecular scale rotary motion: the marriage of overcrowded alkene molecular motor with liquid crystals. Adv. Mater. 34, 2109800 (2022).
Chen, J. et al. Artificial muscle-like function from hierarchical supramolecular assembly of photoresponsive molecular motors. Nat. Chem. 10, 132–138 (2018).
Palagi, S. et al. Structured light enables biomimetic swimming and versatile locomotion of photoresponsive soft microrobots. Nat. Mater. 15, 647–653 (2016).
Li, Q. et al. Macroscopic contraction of a gel induced by the integrated motion of light-driven molecular motors. Nat. Nanotechnol. 10, 161–165 (2015).
Samperi, M. et al. Light-controlled micron-scale molecular motion. Nat. Chem. 13, 1200–1206 (2021).
Iamsaard, S. et al. Conversion of light into macroscopic helical motion. Nat. Chem. 6, 229–235 (2014).
Sato, Y., Sakamoto, T. & Takinoue, M. Sequence-based engineering of dynamic functions of micrometer-sized DNA droplets. Sci. Adv. 6, eaba3471 (2020).
Zhao, Q.-H., Cao, F.-H., Luo, Z.-H., Huck, W. T. S. & Deng, N.-N. Photoswitchable molecular communication between programmable DNA-based artificial membraneless organelles. Angew. Chem. Int. Ed. 61, e202117500 (2022).
Samanta, A., Baranda Pellejero, L., Masukawa, M. & Walther, A. DNA-empowered synthetic cells as minimalistic life forms. Nat. Rev. Chem. 8, 454–470 (2024).
Ramezani, H. & Dietz, H. Building machines with DNA molecules. Nat. Rev. Genet. 21, 5–26 (2020).
Kang, H. et al. Single-DNA molecule nanomotor regulated by photons. Nano Lett. 9, 2690–2696 (2009).
Bandara, H. M. D. & Burdette, S. C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 41, 1809–1825 (2012).
Pang, X., Lv, J.-a, Zhu, C., Qin, L. & Yu, Y. Photodeformable azobenzene-containing liquid crystal polymers and soft actuators. Adv. Mater. 31, 1904224 (2019).
Kamiya, Y. & Asanuma, H. Light-driven DNA nanomachine with a photoresponsive molecular engine. Acc. Chem. Res. 47, 1663–1672 (2014).
Lubbe, A. S., Szymanski, W. & Feringa, B. L. Recent developments in reversible photoregulation of oligonucleotide structure and function. Chem. Soc. Rev. 46, 1052–1079 (2017).
Oscurato, S. L., Salvatore, M., Maddalena, P. & Ambrosio, A. From nanoscopic to macroscopic photo-driven motion in azobenzene-containing materials. Nanophotonics 7, 1387–1422 (2018).
Hong, S. K. & Shu, Y. Photopatterning via photofluidization of azobenzene polymers. Light Adv. Manuf. 3, 65–84 (2022).
Lv, J.-a et al. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature 537, 179–184 (2016).
Ludwanowski, S. et al. Wavelength-gated adaptation of hydrogel properties via photo-dynamic multivalency in associative star polymers. Angew. Chem. Int. Ed. 60, 4358–4367 (2021).
Wu, Y., Ikeda, T. & Zhang, Q. Three-dimensional manipulation of an azo polymer liquid crystal with unpolarized light. Adv. Mater. 11, 300–302 (1999).
Zhou, L., Retailleau, P., Morel, M., Rudiuk, S. & Baigl, D. Photoswitchable fluorescent crystals obtained by the photoreversible coassembly of a nucleobase and an azobenzene intercalator. J. Am. Chem. Soc. 141, 9321–9329 (2019).
Fuentes, E. et al. An azobenzene-based single-component supramolecular polymer responsive to multiple stimuli in water. J. Am. Chem. Soc. 142, 10069–10078 (2020).
Chang, M.-Y., Ariyama, H., Huck, W. T. S. & Deng, N.-N. Division in synthetic cells. Chem. Soc. Rev. 52, 3307–3325 (2023).
Forbes, A., de Oliveira, M. & Dennis, M. R. Structured light. Nat. Photon. 15, 253–262 (2021).
Litchinitser, N. M. Structured light meets structured matter. Science 337, 1054–1055 (2012).
Shen, Y. et al. Optical vortices 30 years on: OAM manipulation from topological charge to multiple singularities. Light: Sci. Appl. 8, 90 (2019).
Opathalage, A. et al. Self-organized dynamics and the transition to turbulence of confined active nematics. Proc. Natl Acad. Sci. USA 116, 4788–4797 (2019).
Fabrini, G. et al. Co-transcriptional production of programmable RNA condensates and synthetic organelles. Nat. Nanotechnol. 19, 1665–1673 (2024).
Fu, H. et al. Supramolecular polymers form tactoids through liquid–liquid phase separation. Nature 626, 1011–1018 (2024).
Dai, Y., You, L. & Chilkoti, A. Engineering synthetic biomolecular condensates. Nat. Rev. Bioeng. 1, 466–480 (2023).
Acknowledgements
This work was supported by the Natural Science Foundation of Shanghai (grant 22ZR1429000 to N.-N.D.), Sichuan Science and Technology Program (2025ZNSFSC0335 to N.-N.D.), National Natural Science Foundation of China (grants 22278264 to N.-N.D. and 22307074 to Q.H.Z.), Sichuan Emei talent plan (2017 to N.-N.D.) and Open Foundation of Shanghai Jiao Tong University Shaoxing Research Institute of Renewable Energy and Molecular Engineering (grant JDSX2022047 to N.-N.D.).
Author information
Authors and Affiliations
Contributions
N.-N.D. supervised the research. N.-N.D. conceived the research and designed the experiments. Q.-H.Z. and J.-Y.Q. performed the experiments. N.-N.D. and Q.-H.Z. analysed the data. N.-N.D. wrote the paper. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Jiawen Chen and Andreas Walther for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–32, Tables 1–7 and captions for Supplementary Videos 1–14.
Supplementary Video 1
UV-induced vesicular dissolution of DNA droplets.
Supplementary Video 2
Vis-induced reassembly of DNA droplets by LLPS.
Supplementary Video 3
Unpolarized Vis-light-fuelled elongation of active DNA droplets and shape recovery.
Supplementary Video 4
Reconstructed 3D confocal images showing 2D liquid slices of the active DNA droplets deformed by linearly polarized Vis light.
Supplementary Video 5
Thermo-induced vesicular dissolution of active DNA droplets.
Supplementary Video 6
Deformation of active DNA droplets into linear patterns under laser illumination.
Supplementary Video 7
Photoinduced fibrillation process from unassociated Y-motifs.
Supplementary Video 8
Fibrillar DNA aggregates spontaneously transform into spherical DNA droplets when Vis illumination stops.
Supplementary Video 9
LLPS of Y-motifs into tactoid-like anisotropic liquid droplets.
Supplementary Video 10
Cell-like extending pseudopodium in active DNA droplets.
Supplementary Video 11
Cell-like division in active DNA droplets.
Supplementary Video 12
Rotation of DNA liquid rods in response to the changes in the polarization direction of the incident light.
Supplementary Video 13
Photofluids for actuating the deformations of ternary Janus droplets.
Supplementary Video 14
Photoactive DNA microfilaments induce flow turbulence in W/O droplets.
Source Data for Supplementary Fig. 3
Statistical source data.
Source Data for Supplementary Fig. 4
Statistical source data.
Source Data for Supplementary Fig. 5
Statistical source data.
Source Data for Supplementary Fig. 7
Statistical source data.
Source Data for Supplementary Fig. 12
Statistical source data.
Source Data for Supplementary Fig. 17
Statistical source data.
Source Data for Supplementary Fig. 21
Statistical source data.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, QH., Qi, JY. & Deng, NN. DNA photofluids show life-like motion. Nat. Mater. 24, 935–944 (2025). https://doi.org/10.1038/s41563-025-02202-0
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41563-025-02202-0
This article is cited by
-
DNA droplets shuffle in the spotlight
Nature Materials (2025)
-
Remodeling and self-healing of individual amyloid tactoids via multiphoton absorption
Nature Communications (2025)
-
Molecular motor-driven reversible liquid-liquid phase separation of supramolecular assemblies
Nature Communications (2025)