Abstract
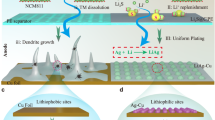
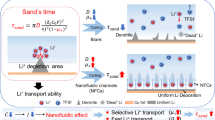
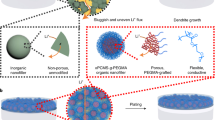
Anode-free lithium (Li) metal batteries are promising candidates for high-performance energy storage applications. Nonetheless, their translation into practical applications has been hindered by the slow kinetics and reversibility of Li plating and stripping on copper foils. Here we report a two-dimensional polyamide (2DPA)/lithiated Nafion (LN) interphase layer for anode-free Li metal batteries. Through molecular engineering, we construct a 2DPA layer with a large conjugated structure and Li-ion adsorption groups that show efficient adsorption, distribution and nucleation of Li ions. 2DPA molecules assembled into two-dimensional sheets are further incorporated with LN to create an ultrathin interphase layer with high-rate, high-capacity Li plating/stripping. These 2DPA/LN layers have higher rate capabilities and maximal energy and power densities compared with alternative polymer interphase layers, enabling the fabrication of an anode-free pouch cell with high performance. Overall, our interphase engineering approach is a promising tool to push the translation of anode-free Li metal batteries based on two-dimensional polymer interphase layers into practical devices, and enable the fabrication of energy storage technologies with high energy and power densities.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All data supporting the findings of this study are available within the article and its Supplementary Information. Source data are provided with this paper.
References
Armand, M. & Tarascon, J. Building better batteries. Nature 451, 652–657 (2008).
He, J. et al. Scalable production of high-performing woven lithium-ion fibre batteries. Nature 597, 57–63 (2021).
Nanda, S., Gupta, A. & Manthiram, A. Anode‐free full cells: a pathway to high‐energy density lithium‐metal batteries. Adv. Energy Mater. 11, 2000804 (2021).
Hobold, G. et al. Moving beyond 99.9% Coulombic efficiency for lithium anodes in liquid electrolytes. Nat. Energy 6, 951–960 (2021).
Dong, L. et al. Toward practical anode-free lithium pouch batteries. Energy Environ. Sci. 16, 5605–5632 (2023).
Xia, Y. et al. Designing an asymmetric ether-like lithium salt to enable fast-cycling high-energy lithium metal batteries. Nat. Energy 8, 934–945 (2023).
Wang, Y. et al. Anode-free lithium metal batteries based on an ultrathin and respirable interphase layer. Angew. Chem. Int. Ed. 62, e202304978 (2023).
Assegie, A., Cheng, J., Kuo, L., Su, W. & Hwang, B. Polyethylene oxide film coating enhances lithium cycling efficiency of an anode-free lithium-metal battery. Nanoscale 10, 6125–6138 (2018).
Hu, A. et al. N, F-enriched inorganic/organic composite interphases to stabilize lithium metal anodes for long-life anode-free cells. J. Colloid Interface Sci. 648, 448–456 (2023).
Liu, H. et al. A scalable 3D lithium metal anode. Energy Storage Mater. 16, 505–511 (2019).
Lu, R. et al. PVDF-HFP layer with high porosity and polarity for high-performance lithium metal anodes in both ether and carbonate electrolytes. Nano Energy 95, 107009 (2022).
Tamwattana, O. et al. High-dielectric polymer coating for uniform lithium deposition in anode-free lithium batteries. ACS Energy Lett. 6, 4416–4425 (2021).
Pyo, S. et al. Lithiophilic wetting agent inducing interfacial fluorination for long‐lifespan anode‐free lithium metal batteries. Adv. Energy Mater. 13, 2203573 (2023).
Sun, Z. et al. Ultra-thin and ultra-light self-lubricating layer with accelerated dynamics for anode-free lithium metal batteries. Energy Storage Mater. 58, 110–122 (2023).
Ouyang, Z. et al. Programmable DNA interphase layers for high-performance anode-free lithium metal batteries. Adv. Mater. 36, 2401114 (2024).
Diaz-Lopez, M. et al. Li2O:Li–Mn–O disordered rock-salt nanocomposites as cathode prelithiation additives for high-energy density Li-ion batteries. Adv. Energy Mater. 10, 1902788 (2020).
Zhu, Y. et al. Lattice engineering on Li2CO3-based sacrificial cathode prelithiation agent for improving the energy density of Li-ion battery full-cell. Adv. Mater. 20, 2312159 (2023).
Genovese, M. et al. Hot formation for improved low temperature cycling of anode-free lithium metal batteries. J. Electrochem. Soc. 166, A3342 (2019).
Liu, P. et al. Ultra-long-life and ultrathin quasi-solid electrolytes fabricated by solvent-free technology for safe lithium metal batteries. Energy Storage Mater. 58, 132–141 (2023).
Ren, Y. & Xu, Y. Recent advances in two-dimensional polymers: synthesis, assembly and energy-related applications. Chem. Soc. Rev. 53, 1823–1869 (2024).
Zeng, Y. et al. Irreversible synthesis of an ultrastrong two-dimensional polymeric material. Nature 602, 91–95 (2022).
Guan, P. et al. High-temperature low-humidity proton exchange membrane with ‘stream-reservoir’ ionic channels for high-power-density fuel cells. Sci. Adv. 9, eadh1386 (2023).
Li, S. et al. A robust all-organic protective layer towards ultrahigh-rate and large-capacity Li metal anodes. Nat. Nanotechnol. 17, 613–621 (2022).
Wang, X. et al. Stress-driven lithium dendrite growth mechanism and dendrite mitigation by electroplating on soft substrates. Nat. Energy 3, 227–235 (2018).
Kim, S. et al. Horizontal lithium electrodeposition on atomically polarized monolayer hexagonal boron nitride. ACS Nano 18, 24128–24138 (2024).
Valadbeigi, Y. & Gal, F. Directionality of cation/molecule bonding in Lewis bases containing the carbonyl group. J. Phys. Chem. A 121, 6810–6822 (2017).
Lu, T. & Chen, F. Bond order analysis based on the Laplacian of electron density in fuzzy overlap space. J. Phys. Chem. A 117, 3100–3108 (2013).
Pei, A. et al. Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 17, 1132–1139 (2017).
Ham, Y. et al. 3D periodic polyimide nano-networks for ultrahigh-rate and sustainable energy storage. Energy Environ. Sci. 14, 5894–5902 (2021).
Yang, Z. et al. Intermolecular hydrogen bonding networks stabilized organic supramolecular cathode for ultra‐high capacity and ultra‐long cycle life rechargeable aluminum batteries. Angew. Chem. Int. Ed. 63, e202403424 (2024).
Li, S. et al. Design and synthesis of a π‐conjugated N‐heteroaromatic material for aqueous zinc–organic batteries with ultrahigh rate and extremely long life. Adv. Mater. 35, 2207115 (2022).
Wang, C. et al. A pyrazine‐pyridinamine covalent organic framework as a low potential anode for highly durable aqueous calcium‐ion batteries. Adv. Energy Mater. 14, 2302495 (2024).
Chen, W. et al. Lithiophilic montmorillonite serves as lithium ion reservoir to facilitate uniform lithium deposition. Nat. Commun. 10, 4973 (2019).
Wu, T. et al. Helmholtz plane reconfiguration enables robust zinc metal anode in aqueous zinc‐ion batteries. Adv. Funct. Mater. 34, 2315716 (2024).
Ge, W. et al. Dynamically formed surfactant assembly at the electrified electrode–electrolyte interface boosting CO2 electroreduction. J. Am. Chem. Soc. 144, 6613–6622 (2022).
Zheng, J. et al. Leveraging polymer architecture design with acylamino functionalization for electrolytes to enable highly durable lithium metal batteries. Energy Environ. Sci. 17, 6739–6754 (2024).
Tan, J., Matz, J., Dong, P., Shen, J. & Ye, M. A growing appreciation for the role of LiF in the solid electrolyte interphase. Adv. Energy Mater. 11, 2100046 (2021).
Xu, Y. et al. Ion-transport-rectifying layer enables Li-metal batteries with high energy density. Matter 3, 1685–1700 (2020).
Wu, Z. et al. Growing single-crystalline seeds on lithiophobic substrates to enable fast-charging lithium-metal batteries. Nat. Energy 8, 340–350 (2023).
Li, N. et al. Reduced-graphene-oxide-guided directional growth of planar lithium layers. Adv. Mater. 32, 1907079 (2020).
Fang, C. et al. Pressure-tailored lithium deposition and dissolution in lithium metal batteries. Nat. Energy 6, 987–994 (2021).
Luo, J., Fang, C. & Wu, L. High polarity poly(vinylidene difluoride) thin coating for dendrite‐free and high‐performance lithium metal anodes. Adv. Energy Mater. 8, 1701482 (2018).
Chen, W. et al. Laser-induced silicon oxide for anode-free lithium metal batteries. Adv. Mater. 32, 2002850 (2020).
Qin, J. et al. Sulfur vacancies and 1T phase-rich MoS2 nanosheets as an artificial solid electrolyte interphase for 400 Wh kg−1 lithium metal batteries. Adv. Mater. 36, 2312773 (2024).
Ye, L. et al. Lithium‐metal anodes working at 60 mA cm−2 and 60 mAh cm−2 through nanoscale lithium‐ion adsorbing. Angew. Chem. Int. Ed. 133, 17559–17565 (2021).
Yu, Z., Cui, Y. & Bao, Z. Design principles of artificial solid electrolyte interphases for lithium-metal anodes. Cell Rep. Phys. Sci. 1, 100119 (2020).
Jung, J. et al. Insights on the work function of the current collector surface in anode-free lithium metal batteries. J. Mater. Chem. A 10, 20984–20992 (2022).
Gao, Y. et al. Low-temperature and high-rate-charging lithium metal batteries enabled by an electrochemically active monolayer-regulated interface. Nat. Energy 5, 534–542 (2020).
Hong, L. et al. Highly reversible zinc anode enabled by a cation-exchange coating with Zn-ion selective channels. ACS Nano 16, 6906–6915 (2022).
Xiao, J. et al. Understanding and applying Coulombic efficiency in lithium metal batteries. Nat. Energy 5, 561–568 (2020).
Louli, A. et al. Diagnosing and correcting anode-free cell failure via electrolyte and morphological analysis. Nat. Energy 5, 693–702 (2020).
Zhang, F. et al. Catalytic role of in-situ formed C-N species for enhanced Li2CO3 decomposition. Nat. Commun. 15, 3393 (2024).
Wu, X., Xiong, X., Yuan, B. & Hu, R. Understanding the phenomenon of capacity increasing along cycles: in the case of an ultralong-life and high-rate SnSe-Mo-C anode for lithium storage. J. Energy Chem. 72, 133–142 (2022).
Wu, N. et al. Suppressing interfacial side reactions of anode‐free lithium batteries by an organic salt monolayer. Small 19, 2303952 (2023).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132, 15 (2010).
Lu, T. & Chen, F. Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem. 33, 580–592 (2012).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Xantheas, S. On the importance of the fragment relaxation energy terms in the estimation of the basis set superposition error correction to the intermolecular interaction energy. J. Chem. Phys. 104, 8821–8824 (1996).
Yurash, B. et al. Towards understanding the doping mechanism of organic semiconductors by Lewis acids. Nat. Mater. 18, 1327–1334 (2019).
Acknowledgements
H.S. acknowledges support from the National Natural Science Foundation of China (22209108) and Fundamental Research Funds for the Central Universities (24×010301678). H.P. acknowledges support from the Ministry of Science and Technology of the People’s Republic of China (2022YFA1203001 and 2022YFA1203002) and National Natural Science Foundation of China (T2321003 and 22335003). Y.W. acknowledges support from the Natural Science Foundation of Shanghai (24ZR1437400). We also acknowledge the BL02U2 station at the Shanghai Synchrotron Radiation Facility for assistance on grazing-incidence wide-angle X-ray scattering measurements.
Author information
Authors and Affiliations
Contributions
H.S. conceived and designed this research project. S.W., Y.W. and Z.O. performed the material synthesis, battery preparation and performance characterization. B.Y. and X. Zhang performed the scanning electronic microscopy and FT-IR measurements. Q.X., Q.C. and S.G. performed the XPS and AFM measurements. S.T. performed the high-resolution cryo-TEM measurements. S.W., Y.W., X. Zhao, P.C., H.P. and H.S. prepared the paper. All authors participated in the data analysis and discussion.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Bing Joe Hwang and Il-Doo Kim for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–37, Tables 1–7 and References.
Supplementary_Video 1
820-mAh 2DPA/LN-Cu||LiFePO4 anode-free pouch cell could power a toy car with a rated power of 8.8 W.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, S., Wang, Y., Ouyang, Z. et al. Molecular engineering of two-dimensional polyamide interphase layers for anode-free lithium metal batteries. Nat. Mater. 24, 1957–1967 (2025). https://doi.org/10.1038/s41563-025-02339-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41563-025-02339-y
This article is cited by
-
Two-dimensional polyamide interphase layers boost anode-free lithium metal batteries
Science China Chemistry (2026)