Abstract
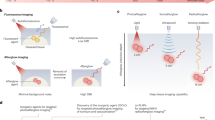
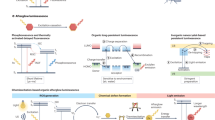
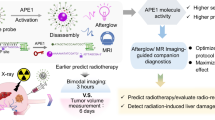
Molecular afterglow imaging is a biomedical modality with high sensitivity and specificity. However, due to the short half-lives of existing afterglow agents, longitudinal imaging often requires multiple on-site reinductions. Here we report a probe with month-long afterglow luminescence and the ability to target a downregulated liver tumour biomarker. This downregulated-biomarker-activatable afterglow probe (DROP) operates through a self-sustainable photoenergy cycling reaction, during which afterglow resonance energy transfer re-excites the afterglow initiator to regenerate singlet oxygen. This process initiates new afterglow resonance energy transfer cycles, extending the afterglow duration to over 40 days. The long afterglow of DROP enables in vivo imaging over 8 h with a single light preinduction, mimicking the imaging process of radioisotopes. Moreover, DROP quickly becomes inactive in healthy liver tissues due to cytochrome P450 enzyme activity, detecting and delineating tumours as small as 1 mm in diameter for complete surgical resection in both murine and rabbit models. Overall, we provide fundamental guidelines to develop radioisotope-mimetic afterglow luminescence probes and highlight the targeting of downregulated biomarkers as a promising approach in cancer theranostics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data generated or analysed during this study are provided as source data or are included in the Supplementary Information. Further data are available from the corresponding authors upon request. Source data are provided with this paper.
References
Hong, G., Antaris, A. L. & Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 1, 0010 (2017).
Lovell, J. F. et al. Porphysome nanovesicles generated by porphyrin bilayers for use as multimodal biophotonic contrast agents. Nat. Mater. 10, 324–332 (2011).
Chen, Y., Wang, S. & Zhang, F. Near-infrared luminescence high-contrast in vivo biomedical imaging. Nat. Rev. Bioeng. 1, 60–78 (2023).
Schnermann, M. J. Organic dyes for deep bioimaging. Nature 551, 176–177 (2017).
Cheng, P. & Pu, K. Enzyme-responsive, multi-lock optical probes for molecular imaging and disease theranostics. Chem. Soc. Rev. 53, 10171–10188 (2024).
Cheng, M. H. Y., Mo, Y. & Zheng, G. Nano versus molecular: optical imaging approaches to detect and monitor tumor hypoxia. Adv. Healthcare Mater. 10, 2001549 (2021).
Nadal-Bufi, F. et al. Fluorogenic platform for real-time imaging of subcellular payload release in antibody–drug conjugates. J. Am. Chem. Soc. 147, 7578–7587 (2025).
Wu, X., Wang, R., Kwon, N., Ma, H. & Yoon, J. Activatable fluorescent probes for in situ imaging of enzymes. Chem. Soc. Rev. 51, 450–463 (2022).
Hyun, H. et al. Structure-inherent targeting of near-infrared fluorophores for parathyroid and thyroid gland imaging. Nat. Med. 21, 192–197 (2015).
Mendive-Tapia, L. & Vendrell, M. Activatable fluorophores for imaging immune cell function. Acc. Chem. Res. 55, 1183–1193 (2022).
Urano, Y. et al. Selective molecular imaging of viable cancer cells with pH-activatable fluorescence probes. Nat. Med. 15, 104–109 (2009).
Wang, F., Zhong, Y., Bruns, O., Liang, Y. & Dai, H. In vivo NIR-II fluorescence imaging for biology and medicine. Nat. Photonics 18, 535–547 (2024).
Owens, E. A., Henary, M., El Fakhri, G. & Choi, H. S. Tissue-specific near-infrared fluorescence imaging. Acc. Chem. Res. 49, 1731–1740 (2016).
Chan, J., Dodani, S. C. & Chang, C. J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 4, 973–984 (2012).
He, S., Cheng, P. & Pu, K. Activatable near-infrared probes for the detection of specific populations of tumour-infiltrating leukocytes in vivo and in urine. Nat. Biomed. Eng. 7, 281–297 (2023).
Waterhouse, D. J., Fitzpatrick, C. R. M., Pogue, B. W., O’Connor, J. P. B. & Bohndiek, S. E. A roadmap for the clinical implementation of optical-imaging biomarkers. Nat. Biomed. Eng. 3, 339–353 (2019).
Gorka, A. P., Nani, R. R., Zhu, J., Mackem, S. & Schnermann, M. J. A near-IR uncaging strategy based on cyanine photochemistry. J. Am. Chem. Soc. 136, 14153–14159 (2014).
Tang, Z. et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 45, W98–W102 (2017).
Uhlen, M. et al. A pathology atlas of the human cancer transcriptome. Science 357, eaan2507 (2017).
Zhou, Y. et al. Tumor biomarkers for diagnosis, prognosis and targeted therapy. Signal Transduct. Target. Ther. 9, 132 (2024).
Miao, Q. et al. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 35, 1102–1110 (2017).
Xu, C. et al. Nanoparticles with ultrasound-induced afterglow luminescence for tumour-specific theranostics. Nat. Biomed. Eng. 7, 298–312 (2023).
Xu, C. et al. A cascade X-ray energy converting approach toward radio-afterglow cancer theranostics. Nat. Nanotechnol. 20, 286–295 (2025).
Wang, Y. et al. In vivo ultrasound-induced luminescence molecular imaging. Nat. Photonics 18, 334–343 (2024).
Pei, P. et al. X-ray-activated persistent luminescence nanomaterials for NIR-II imaging. Nat. Nanotechnol. 16, 1011–1018 (2021).
Jiang, Y. & Pu, K. Molecular probes for autofluorescence-free optical imaging. Chem. Rev. 121, 13086–13131 (2021).
Zhu, J., Zhao, L., An, W. & Miao, Q. Recent advances and design strategies for organic afterglow agents to enhance autofluorescence-free imaging performance. Chem. Soc. Rev. 54, 1429–1452 (2025).
Wu, L. et al. H2S-activatable near-infrared afterglow luminescent probes for sensitive molecular imaging in vivo. Nat. Commun. 11, 446 (2020).
Chen, C. et al. Amplification of activated near-infrared afterglow luminescence by introducing twisted molecular geometry for understanding neutrophil-involved diseases. J. Am. Chem. Soc. 144, 3429–3441 (2022).
Liu, Y., Teng, L., Lou, X.-F., Zhang, X.-B. & Song, G. ‘Four-in-one’ design of a hemicyanine-based modular scaffold for high-contrast activatable molecular afterglow imaging. J. Am. Chem. Soc. 145, 5134–5144 (2023).
Entradas, T., Waldron, S. & Volk, M. The detection sensitivity of commonly used singlet oxygen probes in aqueous environments. J. Photochem. Photobiol. B 204, 111787 (2020).
Thul, P. J. et al. A subcellular map of the human proteome. Science 356, eaal3321 (2017).
Li, Z. et al. Direct aqueous-phase synthesis of sub-10 nm ‘luminous pearls’ with enhanced in vivo renewable near-infrared persistent luminescence. J. Am. Chem. Soc. 137, 5304–5307 (2015).
Xie, C., Zhen, X., Miao, Q., Lyu, Y. & Pu, K. Self-assembled semiconducting polymer nanoparticles for ultrasensitive near-infrared afterglow imaging of metastatic tumors. Adv. Mater. 30, 1801331 (2018).
Chen, W. et al. Near-infrared afterglow luminescence of chlorin nanoparticles for ultrasensitive in vivo imaging. J. Am. Chem. Soc. 144, 6719–6726 (2022).
le Masne de Chermont, Q. et al. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc. Natl. Acad. Sci. USA. 104, 9266–9271 (2007).
Maldiney, T. et al. The in vivo activation of persistent nanophosphors for optical imaging of vascularization, tumours and grafted cells. Nat. Mater. 13, 418–426 (2014).
Zhu, J. et al. A self-sustaining near-infrared afterglow chemiluminophore for high-contrast activatable imaging. Angew. Chem. Int. Ed. 63, e202318545 (2024).
Yan, R. et al. Activatable NIR fluorescence/MRI bimodal probes for in vivo imaging by enzyme-mediated fluorogenic reaction and self-assembly. J. Am. Chem. Soc. 141, 10331–10341 (2019).
Voskuil, F. J. et al. Exploiting metabolic acidosis in solid cancers using a tumor-agnostic pH-activatable nanoprobe for fluorescence-guided surgery. Nat. Commun. 11, 3257 (2020).
Lencioni, R., Cioni, D., Crocetti, L., Pina, C. D. & Bartolozzi, C. Magnetic resonance imaging of liver tumors. Hepatology 40, 162–171 (2004).
Roberts, L. R. et al. Imaging for the diagnosis of hepatocellular carcinoma: a systematic review and meta-analysis. Hepatology 67, 401–421 (2018).
Robinson, P. J. A. Imaging liver metastases—size is important. Cancer Imaging 2, 146–147 (2002).
McNutt, A. T., Li, Y., Meli, R., Aggarwal, R. & Koes, D. R. GNINA1.3: the next increment in molecular docking with deep learning. J. Cheminform. 17, 28 (2025).
Green, O. et al. Opening a gateway for chemiluminescence cell imaging: distinctive methodology for design of bright chemiluminescent dioxetane probes. ACS Cent. Sci. 3, 349–358 (2017).
Gao, Z. et al. An activatable near-infrared afterglow theranostic prodrug with self-sustainable magnification effect of immunogenic cell death. Angew. Chem. Int. Ed. 61, e202209793 (2022).
Ni, X. et al. Near-infrared afterglow luminescent aggregation-induced emission dots with ultrahigh tumor-to-liver signal ratio for promoted image-guided cancer surgery. Nano Lett. 19, 318–330 (2019).
Oushiki, D. et al. Development and application of a near-infrared fluorescence probe for oxidative stress based on differential reactivity of linked cyanine dyes. J. Am. Chem. Soc. 132, 2795–2801 (2010).
Acknowledgements
D.D. thanks the National Natural Science Foundation of China (NSFC, 52225310) and the Fundamental Research Funds for the Central Universities, Nankai University (2122021405) for financial support. Y.Z. thanks the NSFC (22322406) for financial support. K.P. thanks the Singapore National Research Foundation (NRF) (NRF-NRFI07-20210005) and the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE-T2EP30220-0010, MOE-T2EP30221-0004) for financial support. G.F. thanks the NSFC (22595402, 52473300) and Guangdong Provincial Key Laboratory of Luminescence from Molecular Aggregates (2023B1212060003) for financial support. G.-Q.Z thanks the NSFC (52203171) and the Scientific Research Foundation of Hebei Educational Committee (BJK2024192) for financial support. We thank Z. Liang for providing the PDX tissues.
Author information
Authors and Affiliations
Contributions
G.-Q.Z. and G.F. contributed equally to this paper. D.D., Y.Z. and K.P. conceived the study. D.D., Y.Z., K.P., G.-Q.Z. and G.F. designed the experiments. G.-Q.Z. and Z.G. performed the chemical synthesis. G.-Q.Z. performed nanoprobe construction. G.-Q.Z., Z.G. and G.F. performed in vitro characterization. G.F. performed database analysis. L.L. performed molecular dynamics simulation. G.-Q.Z., J.Z. and Z.G. performed in vivo experiments. G.F. and C.X. drew the schematic illustration. K.P., Y.Z., D.D., G.F., C.X. and G.-Q.Z. analysed the data and drafted the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Materials thanks Hak Soo Choi, Guosheng Song and Fan Zhang for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Schemes 1 and 2, Figs. 1–44 and Tables 1 and 2.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, GQ., Feng, G., Xu, C. et al. Radioisotope-mimetic molecular afterglow probe for downregulated cancer biomarker imaging. Nat. Mater. (2026). https://doi.org/10.1038/s41563-026-02507-8
Received:
Accepted:
Published:
Version of record:
DOI: https://doi.org/10.1038/s41563-026-02507-8