Abstract
Deciphering the activity of individual microbes within complex communities and environments remains a challenge. Here we describe the development of microbiome single-cell transcriptomics using droplet-based single-cell RNA sequencing and pangenome-based computational analysis to characterize the functional heterogeneity of the rumen microbiome. We generated a microbial genome database (the Bovine Gastro Microbial Genome Map) as a functional reference map for the construction of a single-cell transcriptomic atlas of the rumen microbiome. The atlas includes 174,531 microbial cells and 2,534 species, of which 172 are core active species grouped into 12 functional clusters. We detected single-cell-level functional roles, including a key role for Basfia succiniciproducens in the carbohydrate metabolic niche of the rumen microbiome. Furthermore, we explored functional heterogeneity and reveal metabolic niche trajectories driven by biofilm formation pathway genes within B. succiniciproducens. Our results provide a resource for studying the rumen microbiome and illustrate the diverse functions of individual microbial cells that drive their ecological niche stability or adaptation within the ecosystem.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All of the raw sequencing data of the MscT have been deposited to the Genome Sequence Archive database with the accession number CRA012211. The genome files of MAGs in the BGMGM, gene annotation files and intermediate files resulting from quality control, benchmarking and other processes have been submitted to the Figshare database at https://figshare.com/articles/dataset/Microbiome_single-cell_transcriptomics_reveal_functional_heterogeneity_of_metabolic_niches_covering_more_than_2_500_species_in_the_rumen/24844344 (ref. 80). Source data are provided with this paper.
Code availability
The main codes and scripts from this study were uploaded to GitHub (https://github.com/J-MimgHui/MscT_codes).
References
Nguyen, C. L. et al. High-resolution analyses of associations between medications, microbiome, and mortality in cancer patients. Cell 186, 2705–2718.e17 (2023).
Albertsen, M. et al. Long-read metagenomics paves the way toward a complete microbial tree of life. Nat. Methods 20, 30–31 (2023).
Lloyd-Price, J. et al. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 569, 655–662 (2019).
Hess, M. et al. Metagenomic discovery of biomass-degrading genes and genomes from cow rumen. Science 331, 463–467 (2011).
Almeida, A. et al. A new genomic blueprint of the human gut microbiota. Nature 568, 499–504 (2019).
Pasolli, E. et al. Extensive unexplored human microbiome diversity revealed by over 150,000 genomes from metagenomes spanning age, geography, and lifestyle. Cell 176, 649–662.e20 (2019).
Valles-Colomer, M. et al. The person-to-person transmission landscape of the gut and oral microbiomes. Nature 614, 125–135 (2023).
Stewart, R. D. et al. Compendium of 4,941 rumen metagenome-assembled genomes for rumen microbiome biology and enzyme discovery. Nat. Biotechnol. 37, 953–961 (2019).
Royo-Llonch, M. et al. Compendium of 530 metagenome-assembled bacterial and archaeal genomes from the polar Arctic Ocean. Nat. Microbiol. 6, 1561–1574 (2021).
Tian, L. et al. Deciphering functional redundancy in the human microbiome. Nat. Commun. 11, 6217 (2020).
Windels, E. M. et al. Bacterial persistence promotes the evolution of antibiotic resistance by increasing survival and mutation rates. ISME J. 13, 1239–1251 (2019).
Lloréns-Rico, V. et al. Single-cell approaches in human microbiome research. Cell 185, 2725–2738 (2022).
Ojala, T. et al. Current concepts, advances, and challenges in deciphering the human microbiota with metatranscriptomics. Trends Genet. 39, 686–702 (2023).
Blattman, S. B. et al. Prokaryotic single-cell RNA sequencing by in situ combinatorial indexing. Nat. Microbiol. 5, 1192–1201 (2020).
Kuchina, A. et al. Microbial single-cell RNA sequencing by split-pool barcoding. Science 371, eaba5257 (2021).
Ma, P. et al. Bacterial droplet-based single-cell RNA-seq reveals antibiotic-associated heterogeneous cellular states. Cell 186, 877–891.e14 (2023).
Xu, Z. Droplet-based high-throughput single microbe RNA sequencing by smRandom-seq. Nat. Commun. 14, 5130 (2023).
Mizrahi, I., Wallace, R. J. & Moraïs, S. The rumen microbiome: balancing food security and environmental impacts. Nat. Rev. Microbiol. 19, 553–566 (2021).
Seshadri, R. et al. Cultivation and sequencing of rumen microbiome members from the Hungate1000 Collection. Nat. Biotechnol. 36, 359–367 (2018).
Xie, F. et al. An integrated gene catalog and over 10,000 metagenome-assembled genomes from the gastrointestinal microbiome of ruminants. Microbiome 9, 137 (2021).
Tong, F. et al. The microbiome of the buffalo digestive tract. Nat. Commun. 13, 823 (2022).
Stewart, R. D. et al. Assembly of 913 microbial genomes from metagenomic sequencing of the cow rumen. Nat. Commun. 9, 870 (2018).
Wilkinson, T. et al. 1200 high-quality metagenome-assembled genomes from the rumen of African cattle and their relevance in the context of sub-optimal feeding. Genome Biol. 21, 229 (2020).
Xue, M.-Y. et al. Investigation of fiber utilization in the rumen of dairy cows based on metagenome-assembled genomes and single-cell RNA sequencing. Microbiome 10, 11 (2022).
Li, X. et al. A unified catalog of 19,251 non-human reference species genomes provides new insights into the mammalian gut microbiomes. Preprint at BioRxiv https://doi.org/10.1101/2022.05.16.491731 (2022).
Solden, L. M. et al. Interspecies cross-feeding orchestrates carbon degradation in the rumen ecosystem. Nat. Microbiol. 3, 1274–1284 (2018).
Parks, D. H. et al. Recovery of nearly 8,000 metagenome-assembled genomes substantially expands the tree of life. Nat. Microbiol. 2, 1533–1542 (2017).
Parks, D. H. et al. GTDB: an ongoing census of bacterial and archaeal diversity through a phylogenetically consistent, rank normalized and complete genome-based taxonomy. Nucleic Acids Res. 50, D785–D794 (2022).
Watson, M. New insights from 33,813 publicly available metagenome-assembled-genomes (MAGs) assembled from the rumen microbiome. Preprint at BioRxiv https://doi.org/10.1101/2021.04.02.438222 (2021).
Louca, S. et al. Function and functional redundancy in microbial systems. Nat. Ecol. Evol. 2, 936–943 (2018).
Escalas, A. et al. Microbial functional diversity: from concepts to applications. Ecol. Evol. 9, 12000–12016 (2019).
Tikhonov, M. Theoretical microbial ecology without species. Phys. Rev. E 96, 032410 (2017).
Taxis, T. M. et al. The players may change but the game remains: network analyses of ruminal microbiomes suggest taxonomic differences mask functional similarity. Nucleic Acids Res. 43, 9600–9612 (2015).
Wang, M. et al. Even allocation of benefits stabilizes microbial community engaged in metabolic division of labor. Cell Rep. 40, 111410 (2022).
Wu, G. et al. Two competing guilds as a core microbiome signature for health recovery. Preprint at BioRxiv https://doi.org/10.1101/2022.05.02.490290 (2022).
Liu, H. et al. Ecological dynamics of the gut microbiome in response to dietary fiber. ISME J. 16, 2040–2055 (2022).
Stacpoole, P. W. & McCall, C. E. The pyruvate dehydrogenase complex: life’s essential, vulnerable and druggable energy homeostat. Mitochondrion 70, 59–102 (2023).
Sun, H.-Z. et al. Multi-omics reveals functional genomic and metabolic mechanisms of milk production and quality in dairy cows. Bioinformatics 36, 2530–2537 (2020).
Cimini, D. et al. Improved production of succinic acid from Basfia succiniciproducens growing on A. donax and process evaluation through material flow analysis. Biotechnol. Biofuels 12, 22 (2019).
Kuhnert, P. et al. Basfia succiniciproducens gen. nov., sp. nov., a new member of the family Pasteurellaceae isolated from bovine rumen. Int. J. Syst. Evol. Microbiol. 60, 44–50 (2010).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Wang, Q. et al. Prediction of prokaryotic transposases from protein features with machine learning approaches. Microb. Genom. 7, 000611 (2021).
Atkovska, K. et al. Energetics and mechanism of anion permeation across formate-nitrite transporters. Sci. Rep. 7, 12027 (2017).
Maertens, G. N. et al. Structure and function of retroviral integrase. Nat. Rev. Microbiol. 20, 20–34 (2022).
Latour, X. The evanescent GacS signal. Microorganisms 8, 1746 (2020).
Li, Q. S. et al. Dietary selection of metabolically distinct microorganisms drives hydrogen metabolism in ruminants. ISME J. 16, 2535–2546 (2022).
Foster, K. R. et al. Competition, not cooperation, dominates interactions among culturable microbial species. Curr. Biol. 22, 1845–1850 (2012).
Friedman, N. et al. Compositional and functional dynamics of the bovine rumen methanogenic community across different developmental stages. Environ. Microbiol. 19, 3365–3373 (2017).
Moraïs, S. et al. The road not taken: the rumen microbiome, functional groups, and community states. Trends Microbiol. 27, 538–549 (2019).
Nayfach, S. et al. New insights from uncultivated genomes of the global human gut microbiome. Nature 568, 505–510 (2019).
Mizrahi, I. et al. Review: the compositional variation of the rumen microbiome and its effect on host performance and methane emission. Animal 12, s220–s232 (2018).
Yu, Z. & Morrison, M. Improved extraction of PCR-quality community DNA from digesta and fecal samples. BioTechniques 36, 808–812 (2004).
Bolger, A. et al. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120 (2014).
Li, D. et al. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015).
Kang, D. D. et al. MetaBAT, an efficient tool for accurately reconstructing single genomes from complex microbial communities. PeerJ 3, e1165 (2015).
Parks, D. H. et al. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res. 25, 1043–1055 (2015).
Uritskiy, G. V. et al. MetaWRAP—a flexible pipeline for genome-resolved metagenomic data analysis. Microbiome 6, 158 (2018).
Bowers, R. M. et al. Minimum information about a single amplified genome (MISAG) and a metagenome-assembled genome (MIMAG) of bacteria and archaea. Nat. Biotechnol. 35, 725–731 (2017).
Orakov, A. et al. GUNC: detection of chimerism and contamination in prokaryotic genomes. Genome Biol. 22, 178 (2021).
Zeng, S. et al. A compendium of 32,277 metagenome-assembled genomes and over 80 million genes from the early-life human gut microbiome. Nat. Commun. 13, 5139 (2022).
Olm, M. R. et al. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. ISME J. 11, 2864–2868 (2017).
Chaumeil, P.-A. et al. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. Bioinformatics 36, 1925–1927 (2019).
Asnicar, F. et al. Precise phylogenetic analysis of microbial isolates and genomes from metagenomes using PhyloPhlAn 3.0. Nat. Commun. 11, 2500 (2020).
Letunic, I. et al. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Hyatt, D. et al. Prodigal: prokaryotic gene recognition and translation initiation site identification. BMC Bioinform. 11, 119 (2010).
Buchfink, B. et al. Sensitive protein alignments at tree-of-life scale using DIAMOND. Nat. Methods 18, 366–368 (2021).
Tatusov, R. L. et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4, 41 (2003).
Kanehisa, M. et al. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 28, 27–30 (2000).
Drula, E. et al. The carbohydrate-active enzyme database: functions and literature. Nucleic Acids Res. 50, D571–D577 (2022).
Li, H. et al. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Lu, J. et al. Metagenome analysis using the Kraken software suite. Nat. Protoc. 17, 2815–2839 (2022).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–3587.e29 (2021).
McGinnis, C. S. et al. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–337.e4 (2019).
Zappia, L. et al. Clustering trees: a visualization for evaluating clusterings at multiple resolutions. GigaScience 7, giy083 (2018).
Korsunsky, I. et al. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 16, 1289–1296 (2019).
Kurtz, Z. D. et al. Sparse and compositionally robust inference of microbial ecological networks. PLoS Comput. Biol. 11, e1004226 (2015).
Bu, D. et al. KOBAS-i: intelligent prioritization and exploratory visualization of biological functions for gene enrichment analysis. Nucleic Acids Res. 49, W317–W325 (2021).
Klopfenstein, D. V. et al. GOATOOLS: a Python library for Gene Ontology analyses. Sci. Rep. 8, 10872 (2018).
Minghui, J. Microbiome single-cell transcriptomics reveal functional heterogeneity of metabolic niches covering more than 2,500 species in the rumen. Figshare https://doi.org/10.6084/m9.figshare.24844344.v1 (2024).
Acknowledgements
We thank all of the members of the Institute of Dairy Science, College of Animal Sciences, Zhejiang University for assistance with sample collection. This work was supported by the National Natural Science Foundation of China (grant no. 32322077 to H.-Z.S.), National Key R&D Program of China (grant no. 2022YFD1301700 and 2023YFE0123100 to H.-Z.S.), Natural Science Foundation of Zhejiang Province (grant no. LR23C170001 to H.-Z.S.) and Leading Innovative and Entrepreneur Team Introduction Program of Zhejiang (grant no. 2021R01012 to Y.W.).
Author information
Authors and Affiliations
Contributions
H.-Z.S. supervised the project and designed the research. H.-Z.S., M.J., S.Z., Y. Tang, X.L. and Y. Tao constructed the BGMGM. M.J., S.Z., Y. Tang and X.L. collected the rumen fluid samples. M.S. performed the RNA-seq experiments with assistance from Y.W. M.J., S.Z., T.Z. and Y. Tao performed the species annotation and gene alignment at the single-cell level. M.J. and S.Z. performed the cluster analysis, cell-type annotation, pathway analysis and pseudo-time analysis. M.J., S.Z., H.C. and H.-Z.S. interpreted the data. M.J., S.Z. and J.X. visualized the results. M.J., S.Z. and M.-Y.X. wrote the paper. H.-Z.S., Y.W. and J.-X.L. revised the paper. All authors read and approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Karthik Raman, James Volmer and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Overall workflow of BGMGM construction.
Workflow for the construction of the Bovine Gastro Microbial Genome Map (BGMGM).
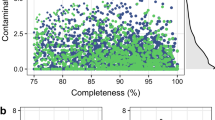
Extended Data Fig. 2 The venn plot of annotated genes and the phylogenic tree of 47 Archaea MAGs.
(A) The Venn plot of BGMGM genes annotated by KEGG database, GO database, CAZy database, and COG database. (B) The phylogenic tree of 47 Archaea MAGs. MAGs: metagenome assembled genomes. BGMGM, bovine gastro microbial genome map.
Extended Data Fig. 3 Microbiome single-cell transcriptomics computational analysis pipeline and performance.
(A) Computational analysis pipeline including microbial pan-genome mapping, taxonomic level-by-level annotation, and functional cluster-based atlas construction. (B) The UMI numbers and unique gene numbers in each sample. UMI, unique molecular identifiers. Each box represents the interquartile range (IQR), in which the middle line represents the median. The whiskers extend to 1.5 × IQR.
Extended Data Fig. 4 Heterogeneity among different functional clusters and species.
(A) The cell and gene filtering steps as well as the benchmarking processes to determine the dimension and resolution values. (B) The UMAP plots for cells of different samples. (C) The UMAP plots for cells of different genera. UMAP, Uniform Manifold Approximation and Projection.
Extended Data Fig. 5 Differences between six functional clusters in the same species analyzed by the Kruskal–Wallis test with Dunn post hoc tests.
(A) The P values of inter-cluster comparison for FGPs in eight species. The UMAP plot presented the functional clusters involved in the analysis. The heat map showed the P values. (B) Dunn post hoc test performed on the FGPs of carbohydrate transport and metabolism. (C) Dunn post hoc test performed on the FGPs of lipid transport and metabolism. (D) Dunn post hoc test performed on the FGPs of amino acid transport and metabolism. FGP: functional gene proportion (the number of functional genes in a certain pathway/the number of all annotated genes in single cell); UMAP, Uniform Manifold Approximation and Projection.
Extended Data Fig. 6 SPIEC-EASI analysis.
The interaction networks of 213 cell units and the interactions between the HSP90+ HMACs—Basfia_succiniciproducens and other associated cell units. SPIEC-EASI, Sparse Inverse Covariance Estimation for Ecological Association Inference.
Extended Data Fig. 7 Carbohydrate metabolic activity analysis.
Average classic carbohydrate metabolic FGPs of 10 sub-functional clusters generated from HMACs. FGPs, functional gene proportions, the number of functional genes in a certain pathway/the number of all annotated genes; HMACs, high metabolic activity cells.
Extended Data Fig. 8 Active cell proportion analysis.
Active cell proportion of 10 sub-functional clusters generated from HMACs in each classic carbohydrate metabolic pathway. HMACs, high metabolic activity cells.
Extended Data Fig. 9 Marker genes and biofilm formation pathway activity analysis of 8 sub-population functional clusters form B. succiniciproducen cells.
(A) The UMAP plot of eiight sub-population functional clusters form B. succiniciproducen cells. (B) Marker genes of eight sub-population functional clusters form B. succiniciproducen cells. (C) Transformational relationships between clusters “Multi signal cells”, “Integrase+ cells”, and “Transposase+ formate/nitrite TCs”. (D) “Biofilm.formation_P” pathway activity and two key gene proportion, n = 200 and 121 biologically independent cells. Data are presented as mean values +/- SEM. Two-side Wilcoxon rank sum test was used for data analysis. UMAP, Uniform Manifold Approximation and Projection.
Supplementary information
41564_2024_1723_MOESM2_ESM.xlsx
Supplementary Table 1 Published ruminant metagenomics datasets used in this study. Supplementary Table 2 Quality of all 55,715 genomes of the BGMGM (54,403 public genomes and 1,312 in-house genomes). Supplementary Table 3 Quality and annotation information for the 47,241 filtered genomes of the BGMGM. Supplementary Table 4 Quality and annotation information for the 13,572 non-redundant genomes of the BGMGM. Supplementary Table 5 Marker gene information for 12 functional clusters and ten sub-functional clusters of HMACs and eight sub-functional clusters of B. succiniciproducens cells. Supplementary Table 6 Average FGPs of 12 functional clusters in each COG pathway. Supplementary Table 7 P values of inter-cluster difference analysis between six functional clusters in 89 species. A Benjamini–Hochberg adjustment was made for multiple comparisons. Supplementary Table 8 Functional gene numbers of 5,636 active cells of HMACs in the classic carbohydrate pathways. Supplementary Table 9 The results of KEGG enrichment analysis. A two-sided Fisher’s exact test was used for data analysis. A Benjamini–Hochberg adjustment was made for multiple comparisons. Supplementary Table 10 Functional gene numbers of B. succiniciproducens cells in the top 14 enriched KEGG pathways. Supplementary Table 11 Functional gene numbers of B. succiniciproducens cells in the classic carbohydrate pathways. Supplementary Table 12 Read number thresholds, cell numbers, average nFeatures and average nCounts of 14 samples. Supplementary Table 13 Annotation results of genes used in classic carbohydrate pathways. Supplementary Table 14 Cell metadata of pseudo-time analysis.
Source data
Source Data Fig. 1
Ingredients and nutrient composition of the total mixed ration fed to the cows.
Source Data Fig. 3
Cell proportion data of core species in Fig. 3c.
Source Data Fig. 5
Unprocessed statistical data of Fig. 5a.
Source Data Fig. 6
Unprocessed statistical data of Fig. 6b and a table of enrichment analysis formula.
Source Data Extended Data Fig. 3
Unprocessed data and summary data for Extended Data Fig. 3b.
Source Data Extended Data Fig. 9
Unprocessed statistical data for Extended Data Fig. 9d.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jia, M., Zhu, S., Xue, MY. et al. Single-cell transcriptomics across 2,534 microbial species reveals functional heterogeneity in the rumen microbiome. Nat Microbiol 9, 1884–1898 (2024). https://doi.org/10.1038/s41564-024-01723-9
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-024-01723-9
This article is cited by
-
Integrative analysis of rumen microbiota and host multi-organ interactions underlying feed conversion efficiency in Hu sheep
Journal of Animal Science and Biotechnology (2026)
-
Rumen microbiota modulates metabolic stress in high-yield dairy cows: insights from early to peak lactation
Microbiome (2026)
-
Understanding microbial ecology and evolution with single-cell genomics
Nature Reviews Genetics (2026)
-
Temporal niche partitioning of bacterial communities shaped by hydrologic fluctuations and drought regimes in karst caves
Ecological Processes (2025)
-
The effects of different silage types on rumen bacteria and metabolites in Tibetan sheep
Microbiome (2025)