Abstract
By acquiring or evolving resistance to one antibiotic, bacteria can become cross-resistant to a second antibiotic, which further limits therapeutic choices. In the opposite scenario, initial resistance leads to collateral sensitivity to a second antibiotic, which can inform cycling or combinatorial treatments. Despite their clinical relevance, our knowledge of both interactions is limited. We used published chemical genetics data of the Escherichia coli single-gene deletion library in 40 antibiotics and devised a metric that discriminates between known cross-resistance and collateral-sensitivity antibiotic interactions. Thereby we inferred 404 cases of cross-resistance and 267 of collateral-sensitivity, expanding the number of known interactions by over threefold. We further validated 64/70 inferred interactions using experimental evolution. By identifying mutants driving these interactions in chemical genetics, we demonstrated that a drug pair can exhibit both interactions depending on the resistance mechanism. Finally, we applied collateral-sensitive drug pairs in combination to reduce antibiotic-resistance development in vitro.
Similar content being viewed by others
Main
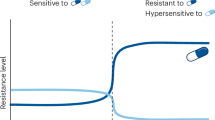
Although antibiotic resistance is increasing at alarming rates1, fewer and fewer novel antibiotics are being approved for clinical use2,3. Importantly, the development or acquisition of resistance to one drug can lead to cross-resistance (XR)4 to other drugs, limiting treatment options. The same processes can also give rise to collateral sensitivity (CS)5 to other drugs due to trade-offs or fitness costs of resistance mechanisms6,7 (Fig. 1a). The principle of CS has been successfully used to reduce the rates of resistance emergence8,9,10,11,12,13,14,15, or even re-sensitize microorganisms to antibiotics16, by combining or cycling CS drug pairs. In an era of diminishing therapeutic options, knowledge of XR and CS is more important than ever.
a, Schematic illustration of the conventional way XR and CS drug interactions are assessed by experimental evolution. Resistant mutants selected by drug 1 are tested for susceptibility to drug 2. The MIC, or 90% inhibitory concentration (IC90), of drug 2 is compared with that of the ancestral strain. b, Schematic illustration of chemical genetic screens with arrayed libraries. Several drugs (drug 1, 2 and so on) are profiled across genome-wide gain-of-function or loss-of-function mutant libraries. The fitness of each mutant is evaluated independently—for example, by measuring colony size. c, XR and CS are associated with chemical genetics profile similarity and dissimilarity, respectively. The s-score (used as a proxy for fitness) of each deletion mutant is plotted for two drugs involved in either XR or CS. If the same mutations make cells more resistant or sensitive to two drugs, cells are more likely to evolve mechanisms that inhibit or promote these exact processes during evolution and become XR to both drugs, whereas the opposite is true for CS.
The most common approach to measure XR and CS is to experimentally evolve resistance to one drug for several lineages and then measure their susceptibility to another drug (Fig. 1a). Our understanding of the underlying mechanism(s) relies on sequencing the genomes of the evolved strains8,17,18,19. Although powerful, this approach is effort, scale and cost heavy. Hence, current knowledge of XR and CS interactions is limited to a few bacteria and a relatively small number of antibiotics8,9,12,16,17,18,19,20,21,22,23,24,25,26. Importantly, experimental evolution probes a limited number of lineages and a small part of the solution space in terms of possible resistance mutations, which strongly depends on the selection pressure applied. This may lead to inconsistencies when assessing drug-pair interactions. Furthermore, experimental evolution leads to the acquisition of numerous mutations that make the identification of causal resistance mechanisms difficult without additional experiments. To facilitate drug susceptibility testing of experimentally evolved strains or to dissect the evolved resistance mechanism(s), adaptations to the original method have been proposed—for example, automation of minimum inhibitory concentration (MIC) measurements26 and phenotypic stratification of evolved strains27,28. Although these adaptations allow for an increase in the number of lineages, chemicals and interactions probed, the genetic space explored for resistance is limited and extensive sequencing, as well as previous knowledge are required to identify the causal resistance mechanisms. Here we set out to overcome these limitations by developing a predictive framework based on the systematic nature of chemical genetics screens.
Chemical genetics involve the systematic assessment of drug effects on genome-wide mutant libraries29,30. Such data have been previously shown to capture information on drug mode of action, resistance and interactions in Escherichia coli31,32,33,34,35,36. Importantly, chemical genetics systematically quantify how each gene in the genome contributes to resistance or susceptibility to a large set of drugs (Fig. 1b). The similarity between chemical genetic profiles for different drugs has been reported to correlate with XR frequency18 and has been used to minimize XR between antimicrobial peptides and antibiotics37. Several years ago we proposed that such chemical genetics data could be used to identify both XR and CS interactions by comparing drug profiles30 (Fig. 1c), expediting the systematic identification of XR/CS interactions and mapping of their underlying mechanisms.
In this study we used available E. coli chemical genetics data31 for 40 antibiotics (Methods) and explored different similarity metrics to identify the one that best discerns between known XR and CS interactions. We applied this metric to all antibiotic drug pairs therein and discovered three times more XR and six times more CS interactions than previously identified, including the reclassification of 116 previously tested drug-pair relationships. We independently validated 8.3% (70/840) of these interactions by experimental evolution with 91% precision (64/70). By integrating all data into a drug-interaction network, we examined the monochromaticity (that is, if a given interaction is exclusively XR or CS) and conservation within antibiotic classes, identifying antibiotic (classes) with extensive XR or CS interactions. All data are available at https://shiny-portal.embl.de/shinyapps/app/21_xrcs. We also used the available chemical genetics data to identify the mutations driving specific interactions, thereby confirming known and resolving new mechanisms. Finally, we showed that newly identified CS pairs used in combination could reduce resistance evolution compared with single drugs. Overall, we present a systematic framework to accelerate XR and CS discovery and mechanism deconvolution, paving the way for the development of rationally designed antibiotic combination treatments.
Results
Building a training set of known XR and CS interactions
To build a training set of known XR and CS interactions, we collected data from four studies that performed experimental evolution in E. coli8,17,18,19. The majority of interactions (78%; 338/429) were only tested in one study. From the 91 antibiotic pairs tested in at least two studies, only one-third (n = 30; 20 neutral, nine XR and one CS) was called uniformly across studies, whereas 56 were called XR or CS interactions in one study but neutral in the other (Fig. 2a). The discrepancy between experimental evolution results could be due to several reasons: selection biases (for example, different selection pressure and number of generations used), slightly different criteria used to define XR and CS (for example, methods and cutoffs used for fitness-effect measurements), low power to call interactions (limited number of lineages tested) and population complexity (resistance or sensitivity assessment is typically done at the population level). We reasoned that most discrepancies were probably due to false negatives (interaction missed and reported neutral in one study), as studies were undersampling the antibiotic-resistance solution space and used different metrics to call interactions. For this reason, we designated drug pairs as XR or CS if they exhibited an interaction in at least one study, even if they were neutral in other(s). In contrast, drug pairs displaying conflicting responses (that is, XR in one study and CS in another) were excluded (n = 5). After comparing drugs for which chemical genetics data are available31, we came up with 206 drug pairs (111 neutral, 70 XR and 25 CS) involving 24 different antibiotics (Source Data Fig. 2), which we used as the training set and ground truth for devising a chemical genetics-based metric to infer XR and CS relationships. The power of chemical genetics is that they probe the impact of loss-of-function mutations of each non-essential gene on the resistance or sensitivity to many drugs. In the chemical genetics data we used, the drug effects on each mutant are represented by s-scores, assessing the fitness of a mutant in one condition compared with its fitness across conditions31,38 (Methods and Supplementary Table 1).
a, The overlap between published XR and CS interactions from four existing datasets8,17,18,19 is low, even when directionality is not taken into account. b, A devised metric derived from chemical genetics profile similarity, OCDM, can robustly discern between known XR, CS and neutral interactions. False detection rate-adjusted P values were obtained from a two-sided Mann–Whitney U-test. The box boundaries represent the first and third quartiles, with the median indicated. The whiskers extend to the furthest data points within 1.5× the interquartile range. c, ROC curves for the classification of XR (positive class) versus non-XR (negative class) and CS (positive class) versus non-CS (negative class). Each OCDM cutoff represents a point on the curve and is associated with a true-positive rate and a false-positive rate. The OCDM cutoffs chosen for XR and CS interactions are depicted with a closed circle. d, New XR, neutral and CS pairs inferred by chemical genetics using the OCDM cutoff expand the currently known XR and CS interactions in E. coli by two- and fourfold, respectively. This difference further increases if we take into account drug pairs for which the interaction is inferred differently from previous studies (Extended Data Fig. 2). Note that known interactions (n = 420 total) include drug pairs for which there is no available chemical genetics data.
Chemical genetics profile concordance identifies XR and CS
Using our training set, we hypothesized that XR drugs should share resistance mechanisms (XR) and thus have concordant chemical genetic profiles, as previously suggested for a subset of XR pairs (n = 36)18. The opposite should be true for CS pairs, as mutations causing resistance to one drug would sensitize cells to another, leading to discordant chemical genetics profiles for the two drugs (Fig. 1c). We first tested whether different correlation-based metrics from chemical genetics data could discriminate between known XR, CS or neutrality (Methods) but all performed poorly (area under the curve (AUC) for the receiver operating curve (ROC), 0.52–0.67; Extended Data Fig. 1a). We reasoned that the noise generated by the high proportion of neutral phenotypes in the chemical genetics data31 was compromising performance. To overcome this, we used six features based only on extreme s-scores per condition: the sum and count of positive concordant s-scores, negative concordant s-scores and total discordant s-scores (Methods). We then trained decision tree models with these features for each drug pair. The trained classifier performed well, with the F1 score, recall, precision and ROC AUC consistently exceeding 0.7 (Extended Data Fig. 1b). To avoid overfitting of a model based on a suboptimal training dataset of XR/CS (Fig. 2a), we aimed to interpret the model instead of applying it directly on our test dataset. We learned from decision tree attributes (Extended Data Fig. 1c) that the sum and count of concordant negative s-scores are the most informative features, followed by the sum of discordant s-scores. In addition, if the count of concordant negative s-scores was higher than the median count of concordant hits across all drug pairs (n = 7), the level of discordance would lose importance for classifying interactions. Based on this information we came up with the outlier concordance–discordance metric (OCDM), which discriminated previously reported CS and XR interactions from neutral ones (ROC AUC = 0.76 and 0.73, respectively; Fig. 2b,c, Source Data Fig. 2 and Methods), and selected the cutoff for extreme s-scores based on the OCDM performance (Extended Data Fig. 1d). We then used the OCDM cutoffs (Fig. 2c and Methods) to classify all possible interactions between the 40 antibiotics within the chemical genetics data31 (the confusion matrix is shown in Extended Data Fig. 1e). This yielded 634 new drug-pair relationships (313 XR, 196 CS and 125 neutral), expanding the number of known XR and CS interactions by two and four times, respectively (Fig. 2d and Supplementary Table 2).
Based on the OCDM, drug pairs were inferred as XR if there was high concordance in the mutant profiles despite any discordance signal. In contrast, CS relationships required not only high discordance, but also no concordance signal in the chemical genetics profiles. The priority in concordance when defining interactions reflects the fact that XR-conferring mutations will dominate over CS mutations when a heterogeneous population, evolved in the first drug, is treated with a second drug. Overall, our metric does not classify interactions as exclusively XR or CS but rather reflects the frequency/strength of concordance or discordance of chemical genetics profiles of thousands of gene knockout mutants. In terms of previously measured drug pairs (n = 206), our metric agreed with 90 and disagreed with 116 of the previously identified interactions. 85/116 were previously identified as neutral interactions (Extended Data Fig. 2a–c) and we reasoned that these may be potential false negatives—akin to what was observed when comparing drug pairs across studies (Fig. 2a). This increased the total number of inferred drug-pair relationships to 840 (404 XR, 267 CS and 169 neutral) and expanded the number of known XR and CS interactions by three and six times, respectively (Extended Data Fig. 2d). A user-friendly Shiny app available at https://shiny-portal.embl.de/shinyapps/app/21_xrcs allows the user to browse the XR and CS interaction data per drug pair, class-based pair or genes of interest and includes views of drug class interactions.
Chemical genetics-based metric accurately infers XR and CS
To benchmark our chemical genetics-based metric (OCDM) and cutoff decisions, we selected a subset of 38 newly inferred and 32 previously tested drug pairs (for 21/32, we predicted a different interaction than one previously reported) and measured their interactions using experimental evolution. In our setup we evolved resistance to 23 antibiotics in 12 lineages for up to about 50 generations (population bottleneck, approximately 2 × 106 cells; Methods) and tested resistant lineages for changes in susceptibility to a second antibiotic (Supplementary Table 3, Fig. 3a and Methods). Drug pairs were chosen to cover a wide OCDM range and to have low initial MICs to be able to evolve several-fold resistance. The number of antibiotic pairs belonging to the same chemical class was limited (n = 3) to avoid inflating the prediction accuracy of XR predictions, as same-class drug pairs are likely to share resistance mechanisms. Evolving resistance to both drugs of each pair allowed us to assess the (bi)directionality of interactions, something that the OCDM score cannot assess. By definition, XR interactions are bidirectional and failure to detect them in both ways in experimental evolution experiments exemplifies the limitations of the method. In contrast, CS interactions can be directional, as resistance mechanisms for each drug of the pair can be different and not bear a fitness cost to the other drug. Hence, most of the previously detected CS pairs have been unidirectional. To decrease false negatives (that is, the failure to detect an interaction), we evolved resistance to a large number of lineages (n = 12), probed interactions in both directions and avoided strict cutoffs on the number of lineages required to exhibit an interaction to call drug pairs CS or XR (one was enough). As in our OCDM score, we considered XR interactions dominant to CS and non-monochromatic drug pairs (with lineages exhibiting both XR and CS) were deemed to be XR.
a, Schematic of benchmarking conducted for 70 drug pairs by experimental evolution and IC90 measurements. Twelve lineages were evolved in parallel for five passages in increasing concentrations of 23 antibiotics. At each passage, the culture growing at the highest concentration was transferred to a new antibiotic gradient. The IC90 of the final resistant population was then measured for all lineages in the relevant antibiotics. b–d, Heatmaps of 70 new and known drug-pair interactions, split depending on whether they were inferred as CS (b), neutral (c) or XR (d). Interactions were tested in both directions, with the drug for which selection occurred shown first and the drug for which MIC/IC90 was tested shown second. In each interaction, all tested lineages are shown (n = 9–12). Coloured boxes denote the interaction observed for a given lineage. The three columns on the right of the lineage results represent the summary for all lineages. We considered an interaction as validated if the log2-transformed IC90 fold change was >1 for XR and <−1 for CS in any direction tested for at least one lineage compared with the wild type. An interaction of a drug pair was deemed to be XR if there was at least one lineage showing XR despite any CS for other lineages. Interaction monochromaticity (that is, whether the interaction is exclusively CS or XR; neutral lineages do not affect this call and were labelled as not applicable (N/A)) and directionality (drug pair interacting consistently in both directions) are shown. Interactions referred to as reclassified in the text are those for which our inference and validation agree but previous reports have reported differently. The interaction in red (least monochromatic interaction) is used in Fig. 5 to understand the mechanisms in play. The interactions in bold are used later in Fig. 6 to test resistance evolution in drug combinations. The interaction in italics (drug pair 14), which was conflicting across studies (XR in one study and CS in another), has been inferred and validated to be CS.
In total, we validated all but six of the inferred interactions, which amounts to a validation rate of 91.4%. Not only did we confirm all interactions for which previous studies and our metric agreed (n = 11) but also 18/21 interactions for which our predictions contradicted previous studies (Fig. 3b–d). This implies that several more of the 116 interactions that the OCDM metric classified differently from previous reports may be correct (Extended Data Fig. 2a–d). This high validation rate could be positively influenced by loss-of-function mutations typically dominating short evolution experiments, as the one we performed here (50 generations), and the OCDM score being based on chemical genetics data of an E. coli single-gene deletion library. To test whether longer experimental evolution would influence precision, we continued the evolution for a subset of drugs for 100 generations and probed 14 drug pairs for XR and CS, including three drug pairs that our shorter evolution experiment could not validate. Eleven of the 14 interactions agreed with the chemical genetics-based inferences despite individual lineages changing interactions with time and overall CS interactions decreasing during longer evolution (Extended Data Fig. 2e). Overall, chemical genetics could capture the results of experimental evolution regarding XR and CS well, although observed frequencies change with duration and strength of selective pressure.
The four published studies contained only 25 CS interactions. Here we inferred and validated 23 further CS interactions as well as two known ones (Fig. 3b). The majority of the validated CS interactions (n = 19/25) were bidirectional. The two non-monochromatic interactions that exhibited single instances of XR were classified as XR per our definition (Fig. 3b). This illustrates the power of chemical genetics to identify new CS interactions, especially the rare bidirectional ones, which are the most promising for cycling/combination therapies8,9,10,11,12,13,14,15. In contrast to CS drug pairs, about one-third of the tested XR pairs (n = 11/38), including those that were previously known, were non-monochromatic (Fig. 3d)—that is, some evolved lineages were sensitive, instead of resistant, to the second antibiotic. We failed to detect the expected bidirectionality in nine XR cases and failed to detect the interaction after 50 generations of experimental evolution in four further cases (Fig. 3c); however, we detected the XR interaction for three out of four cases after 100 generations (Extended Data Fig. 2e). Overall, the discovery of XR/CS using evolution experiments strongly depends on the experimental design and, given that frequencies change with experimental setup, calls are sensitive to strict thresholds and low numbers of lineages probed.
Antibiotic classes with extensive XR or CS
In contrast to other studies looking into CS and XR, where mostly one antibiotic per class is tested, here we could assess antibiotic class behaviours, as for some classes, several members were profiled in the chemical genetics data31. Antibiotics from the same class exhibited exclusively XR interactions, as they largely shared the mode of action and mechanisms of resistance (Discussion). In contrast, as previously reported39, antibiotics of different chemical classes exhibited both XR and CS interactions (Fig. 4a), the former often driven by promiscuous resistance mechanisms (for example, efflux pumps) and the latter by mutations that lead to modifications of the outer-membrane composition (Extended Data Fig. 3). We next investigated whether antibiotic classes behaved coherently, that is, whether members of two classes interacted predominantly in the same way. Although this was true for antibiotic classes with members that share cellular target(s) and/or transport mechanisms (for example, tetracyclines and macrolides), this was less the case for classes with distinct targets (β-lactams) or transport mechanisms (quinolones of different generations; Fig. 4c). Interestingly, protein synthesis-inhibitor classes did not only act coherently but also exhibited mostly XR interactions between them (Fig. 4b), with the exception of aminoglycosides, which are known to be CS with drugs of different classes8,17,19.
a, Interactions between members of the same antibiotic class (within class) are exclusively inferred as XR. The within-class group includes classes with more than one member probed—that is, β-lactams, aminoglycosides, quinolones, macrolides, tetracyclines and sulfonamides. b, Overview of all inferred and known drug interactions in E. coli at the class level. When a class has only one representative the antibiotic is named and shown in grey. The heat map sums XR and CS interactions across drug classes inferred by the OCDM metric. Within-class interactions are not displayed in the plot but are all exclusively classified as XR. Antibiotics are grouped according to their modes of action. Dot size represents the count of interactions between classes (or single antibiotics). c, Coherency of interactions of each class with all other classes—that is, if all members of the class interact the same with other classes—calculated as the sum of the absolute differences between the number of XR and the number of CS interactions with each other class normalized to the number of drugs in the class. The higher the number, the more coherently the class is behaving. d, Interaction preference of each class (single- or multi-membered), calculated as the log2-transformed ratio of the number of CS and XR interactions with all other antibiotics from other classes. Antibiotic classes with a ratio of >0 are considered predominantly CS (n = 8), whereas those with a ratio of <0 as predominantly XR (n = 12). Antibiotic classes in bold are classes with more than one antibiotic tested.
Besides aminoglycosides, the only other class reported to be enriched in CS interactions are polymyxins8,17. In addition to these two classes and nitrofurantoin, for which CS interactions have been reported before17, we identified sulfonamides and several single drugs (fosfomycin, rifampicin and tunicamycin) with extensive CS interactions (Fig. 4b,d). Sulfonamides were largely CS to macrolides and β-lactams, driven by lipopolysaccharide (LPS)- and nucleotide biosynthesis-related mechanisms (Fig. 4b and Extended Data Fig. 3a). In contrast, protein-synthesis inhibitors (apart from aminoglycosides) were enriched in XR interactions, probably because of shared efflux resistance mechanisms (AcrAB–TolC; Fig. 4b,d and Extended Data Fig. 3b).
Chemical genetics unravel CS and XR mechanisms
Understanding the mechanisms of XR and CS interactions from sequences of experimentally evolved strains is challenging, as passenger mutations occur in parallel to the causal mutation(s), and indirect mutations can also affect the expression/activity of causal resistance elements. The situation is even less obvious for CS interactions, for which very few mechanisms are known to date7,17,19,26. Chemical genetics make it easier to disentangle causality as all genes contributing to resistance or sensitivity to a certain drug are identified. To explore this, we first investigated how known CS interactions were represented in chemical genetics. For example, the decrease in proton motive force across the inner membrane decreases aminoglycoside uptake and makes cells more resistant to aminoglycosides, but also collaterally sensitive to drugs whose efflux is driven by proton motive force-dependent pumps, such as AcrAB–TolC17,19. Mutations in trkH, which encodes a proton-potassium symporter, were previously shown to cause this phenotype, in particular for the CS interaction between the aminoglycoside tobramycin and nalidixic acid or tetracycline17,39. The trkH mutant, as well as mutants in subunits of the respiratory complexes17,39, indeed exhibited discordant s-scores for these known CS drug pairs in chemical genetics (Extended Data Fig. 4a). Using the same logic, we tried to deduce the unknown mechanism of the recently described CS interaction between cefoxitin and novobiocin26. Genes involved in adding polarity to the LPS core—waaG, waaP and waaQ—were strongly discordant for this drug pair, leading to cefoxitin resistance and novobiocin sensitivity (Extended Data Fig. 4b). The outer-membrane penetration of novobiocin, a large lipophilic antibiotic, is known to be affected by LPS modifications40,41. At the same time, these mutations lower the levels of the outer-membrane porins OmpC and OmpF42, allowing less cefoxitin and other cephalosporins to enter the cell43.
Drug interactions can be non-monochromatic, as multiple resistance mechanisms exist for a given drug. Given that chemical genetics systematically explore the mutational space (of single loss-of-function mutations), we assumed that they should capture the dynamics of such interactions better. To assess this, we focused on XR drug pairs that exhibited non-monochromaticity in our validation experiment (n = 11/38; Fig. 3d). Antibiotic pairs with non-monochromatic XR interactions exhibited significantly stronger discordance scores in chemical genetics than drug pairs with monochromatic XR (P = 1.00 × 10−5; Extended Data Fig. 4c). Hence, chemical genetics can capture monochromaticity of XR interactions and potentially identify the antibiotic pairs that can evolve both XR and CS relationships (Extended Data Fig. 4d–g). We then investigated the most non-monochromatic pair in more detail, that is, tetracycline and azithromycin, which showed XR, CS and neutral interactions in four, six and two lineages, respectively (Fig. 3d). For each of our 12 tetracycline-evolved lineages, we measured changes in susceptibility to both antibiotics at each of the ten passages (Fig. 5a and Methods). Almost all lineages exhibited increased neutrality with time and as resistance to tetracycline increased, except for three lineages (lineages 1, 4 and 12), which evolved low resistance to tetracycline and remained CS to azithromycin (Fig. 5a). First, and as noted earlier, this could partially explain the low rates of CS and XR discovery in previous studies (Fig. 2a), given that XR and CS is typically assessed using final populations with high resistance to one drug. Second, it suggests that with time cells evolve more specific resistance mechanisms—for example, target- compared with intracellular concentration-related mechanisms.
a, Changes in azithromycin susceptibility during the evolution of 12 lineages in tetracycline (100 generations, Methods). Resistance levels of 12 lineages to both antibiotics are shown for days 2, 3, 5, 7 and 10. Lineages are grouped according to whether they exhibited CS, neutrality or XR on day 5 (same as Fig. 3d). Dashed lines indicate the neutral threshold. b, Chemical genetic profiles of the E. coli deletion library in tetracycline and azithromycin31. Mutants with concordant (XR-related) and discordant (CS-related) profiles are highlighted. Dots in grey represent mutants that do not have s-scores within the 3% extreme cutoff for both drugs. Lines at x = 0 and y = 0 are shown to separate concordant and discordant zones of the plot. c, Mutations of lineage 11 during evolution. Genome sequencing of the lineage population reveals a succession of two point mutations in genes that both lead to CS—first in hldE, which is then replaced by mutations in waaF, a slightly less detrimental gene for azithromycin resistance according to chemical genetics data in b. For the other 11 lineages see Extended Data Fig. 5. d, The fold change in tetracycline and azithromycin IC90 of knockout mutants compared with the wild type confirms that both hldE and waaF contribute to resistance to tetracycline and sensitivity to azithromycin, whereas ompF deletion leads only to resistance to tetracycline; n = 6 biological replicates. e, Tetracycline uptake is reduced in a waaF deletion (ΔwaaF) mutant. Tetracycline fluorescence was measured in cell pellets and the signal was normalized to the optical density at 600 nm (OD600nm); n = 3–6 biological replicates. d,e, Data are the mean ± s.e.m. f, OmpF, a major tetracycline importer, is the most downregulated protein in ΔwaaF42. FC, fold change.
To understand the mechanisms driving changes in the tetracycline–azithromycin relationship over time, we sequenced all 12 lineage populations from days 3, 5 and 7 (Extended Data Fig. 5). Lineages with neutral interactions carried either point mutations in tetracycline target genes (for example, lineage 3 with rpsJ V57L, coding for the S10 ribosomal protein44) or a combination of CS and XR strains in the population (for example, linage 7 with mutations in hldE and marR; Fig. 5a and Extended Data Fig. 5). Mutations in marR, which encodes a repressor of efflux pumps in E. coli and is a known modulator of antibiotic resistance45,46, were behind all XR interactions observed in different lineages (lineages 2, 5, 7 and 10; Fig. 5a and Extended Data Fig. 5). This was consistent with marR deletion (ΔmarR) increasing resistance to both drugs in chemical genetics data (Fig. 5b). In contrast, all lineages with stable and strong CS interactions had promoter or deletion mutations in waaD (Extended Data Fig. 5), one of the most sensitive mutants to azithromycin and resistant to tetracycline in chemical genetics data31,47 (Fig. 5b). Lineages that were initially CS but became neutral (lineages 8, 9 and 11) carried initially strong CS mutations on waaD or hldE (both are involved in synthesis of the ADP–heptose precursor of core LPS), which were then replaced by strains with mutations in genes with milder CS or XR phenotypes, such as waaF and marR (Fig. 5b,c and Extended Data Fig. 5). We confirmed the slightly milder CS (lower azithromycin sensitivity) for the waaF deletion mutant (ΔwaaF), a gene encoding a protein that adds the second heptose sugar to the LPS inner core, compared with the hldeE and waaD deletion mutants (Fig. 5d). We postulated that the increased tetracycline resistance of all LPS core mutants is due to reduced uptake compared with the wild type and confirmed this by measuring intracellular tetracycline fluorescence in ΔwaaF cells (Fig. 5e and Source Data Fig. 5e). This lower intracellular tetracycline concentration is probably due to low OmpF levels in ΔwaaF cells (Fig. 5f)42, as OmpF is the major tetracycline importer43,48,49. This is in agreement with chemical genetics data, where ΔompF is tetracycline-resistant but not azithromycin-sensitive (Fig. 5b,d). Hence, loss-of-function mutations in waaF (or in other LPS core genes such as hldE, waaD and waaP) reduced the OmpF levels in the outer membrane and increased tetracycline resistance. At the same time, cells became more sensitive to azithromycin (and macrolides) because their outer membrane became less polar and thereby more permeable to hydrophobic antibiotics50.
Overall, we confirmed that chemical genetics data can pinpoint CS and XR mechanisms that emerge and get selected during experimental evolution, thereby helping us to rationalize the dynamics of non-monochromatic antibiotic interactions.
Combining CS antibiotic pairs reduces resistance evolution
Combination, sequential use and cycling of CS drug pairs reduce the rate of resistance evolution8,9,10,11,12,13,14,15 and re-sensitize resistant strains16 in laboratory settings. This has also been observed for a Pseudomonas aeruginosa infection in clinics23. Considering the therapeutic potential of CS antibiotic combinations, we tested the degree to which our newly identified CS pairs reduced resistance evolution in combination when compared with single drugs (Fig. 6a). We selected four CS pairs, two neutral pairs and one XR pair involving nine antibiotics. We evolved seven E. coli lineages to single drugs or combinations (using a 1:1 ratio compared with drug MICs) for seven days and measured the IC90 of the evolved populations (Fig. 6a and Methods). For each antibiotic combination, we calculated 2,401 evolvability indices (74 combinations), that is, the degree by which resistance to any of the single drugs increases (log2(evolvability index) > 0) or decreases (log2(evolvability index) < 0) in the drug combination (Methods)21. As expected, lineages evolved in the presence of the ceftazidime–ciprofloxacin XR combination reached higher resistance to each drug compared with lineages evolved with single antibiotic treatments (Fig. 6b and Source Data Fig. 6). In contrast, most lineages treated with CS or neutral combinations evolved lower resistance than those treated with single antibiotics (Fig. 6b). The strongest reduction in resistance evolution occurred for combinations of bidirectional CS pairs (Figs. 3c and 6b). For example, six of seven lineages evolved full resistance towards mecillinam alone (256-fold increase in MIC), whereas the combination of mecillinam with nitrofurantoin or levofloxacin led to almost no mecillinam resistance (average fold change in IC90 < 2). For the cefoxitin–levofloxacin pair, resistance evolved in combination was lower just for cefoxitin (Fig. 6b and Extended Data Fig. 6), despite the pair showing bidirectional CS during experimental evolution (Fig. 3c). Together, we demonstrate that reciprocal CS antibiotic pairs hold a great potential for diminishing resistance evolution when used in combination.
a, Experimental design. After evolving resistance to single antibiotics or their combination (seven lineages for each, passaged every 24 h for 7 d; 70 generations in total), the IC90 of both antibiotics was determined for the evolved mutants. In each passage mutants growing (coloured yellow) at the highest concentration (well marked by a thick circle) were transferred (Methods). b, The measured IC90 values were used to calculate the evolvability index (equation (2), Methods; data using slightly different original evolvability indices (equation (3), Methods) in Extended Data Fig. 6a). The red line represents the cutoff (log2(evolvability index) = 0; the evolvability index was log2-transformed to make data symmetrical) below which the antibiotic pair is considered to reduce resistance evolution compared with single antibiotics. Red dots on the violin plots represent the median. The box boundaries represent the first and third quartiles, with the median indicated. The whiskers extend to the furthest data points within 1.5× the interquartile range. Non-XR antibiotic combinations led to lower collective resistance and in the case of reciprocal CS to lower evolvability indices and lower resistance to each of the antibiotics combined (Extended Data Fig. 6b).
Discussion
A better understanding of how resistance to one antibiotic limits treatment with others (XR) or opens new opportunities (CS) is imperative in the context of the ongoing antimicrobial resistance crisis. In the last decade such drug interactions have been assessed for several pathogens8,12,16,17,18,21,22,23,24,25,51. However, the main detection method, experimental evolution, has limitations. First, it has low sensitivity, which leads to different studies reporting different interactions for the same drug pairs in the same species (Fig. 2a). This is because only limited numbers of lineages and resistance mechanisms are probed. What further augments the problem is that resistance mechanisms largely depend on the amount and time of selective pressure applied, as we show for the tetracycline–azithromycin pair (Fig. 5a) and for the 13 further drug pairs probed for both 50 and 100 generations (Extended Data Fig. 2e). In addition to these inherent limitations each study uses different selection pressures, metrics and number of lineages to assess interactions. Although within-species comparisons are possible when the metric and selection pressure are standardized52, cross-species comparisons become challenging as it is unclear whether the differences in interactions stem from biological (different resistance mechanisms) or technical reasons (false negatives, more difficult to standardize selective pressure across species). Second, experimental evolution is laborious and limits the number of drug pairs that can be tested. As a result, the monochromaticity of interactions (especially for drug classes) has been challenging to assess properly in the past. Last, it is very hard to identify the underlying mechanism for CS and XR interactions by only sequencing resistant lineages and without additional tailored experiments.
By assessing the impact of thousands of individual mutations at once on resistance or sensitivity to different drugs, chemical genetics can bypass most of these limitations. As we show here chemical genetics offer a way to systematically and quantitatively assess all chromosomal resistance mechanisms (independent of selective pressure) and can dramatically increase the throughput of bacterial species and drugs tested. In addition, it can provide insights into how monochromatic or conserved such interactions are as well as a basis to dissect the driving mechanisms. As proof-of-principle we focused on published chemical genetics data from E. coli31 because of the large number of antibiotics screened at different concentrations and the extensive benchmarking. In the future similar analyses can be expanded to other available datasets in the same or other species34,47,53,54,55,56, but the OCDM metric may need to be fine-tuned and/or retrained, especially if the fitness metric and dynamic range of the data are different. Such datasets will inevitably increase with time as genome-wide mutant libraries are becoming available for tens of species and even more strains57,58; these can be arrayed or pooled29,59 and constructed by targeted deletions60,61,62, transposon insertions59,63 or CRISPRi knockdowns53,64. Including such libraries will allow probing of the role of essential genes and/or gene overexpression when mapping antibiotic resistance and XR/CS relationships. An obvious limitation of our current metric is that it is based on single loss-of-function (deletion) mutations of non-essential genes. During evolution (in the laboratory or in patients) resistance does not only arise by frequent loss-of-function mutations in non-essential genes but also by less frequent gain-of-function mutations (via point mutations, insertions or duplications) and by mutating essential genes (for example, antibiotic target). Moreover, epistatic relationships between multiple mutations can affect both resistance and XR/CS to other drugs. As global epistasis maps65 become more common in bacteria in the future, such data could make XR and CS inferences even more robust.
In this study we devised an approach and metric to map CS and XR in E. coli using available chemical genetics data for 40 antibiotics. We thereby increased the number of known interactions by several-fold, validated previous conflicts in literature and proposed different interactions for 116 drug pairs reported mostly neutral (n = 85) by single studies before (18 were further validated by experimental evolution). Beyond this we obtained unique insights into within-class interactions, unravelling that all antibiotic classes are dominated by XR interactions between their members. Although this is largely expected, some classes have members with non-overlapping targets and/or resistance mechanisms. Specifically for β-lactams, their use in combination has been reported to constrain resistance evolution during fast-switching regimens66 or for specific pairs and resistance mechanisms67. Moreover, we identified many new bidirectional CS interactions and used a handful to show that the evolution of antibiotic resistance to combinations of such antibiotics is harder. In the past evolutionary variability and non-monochromaticity of CS interactions has been identified as a bottleneck for their use in clinics68,69. It remains to be seen if the ability to identify monochromatic and bidirectional CS drug pairs alleviates some of these limitations. Finally, we mechanistically rationalized CS interactions and explained why some drug interactions can be non-monochromatic. In the case of tetracycline–azithromycin, the mechanisms that played a role in experimental evolution were a small subset of the possible mechanisms revealed by chemical genetics. This is probably because only 12 lineages were probed but also likely to be due to the fitness costs of some of these resistance mechanisms. Interestingly, the interaction changed non-monotonically over time and longer/stronger selection on one drug (tetracycline) led to more neutral interactions with the second drug (azithromycin). This means that long-term, bacterial populations may opt for target mutations or low/neutralized fitness-cost resistance mechanisms, neutralizing also CS/XR interactions. Hence, fast-switching or combinatorial treatments may be more efficient than sequential antibiotic treatments for CS drug pairs.
The increased ability to map XR and CS interactions between drugs opens the path for future expansion of such endeavours to non-antibiotics with antibacterial or adjuvant activity70,71,72 and to probing interactions in different environments—such as in bile, different pHs73, urine media, biofilms74 or gut microbiome communities—as fitness costs are known to change with the environment75. Moreover, the systematic nature of chemical genetics limits false negatives and metric biases and can allow for comprehensive comparisons across species and strains using corresponding genome-wide mutant libraries. Cross-species studies have been conducted previously to map drug synergies and antagonisms35,76. Knowledge on how drugs interact at multiple levels—resistance evolution, efficacy, long-term clearance effects77 and host cytotoxicity—will open the path for designing better combinations for the clinical setting.
Methods
Data sources and preprocessing
The E. coli chemical genetics data were obtained from a previous study31 in which the fitness of 3,979 non-essential single-gene knockout mutants and essential gene hypomorphs was evaluated in 324 different conditions (114 unique stresses and drugs tested in different concentrations). Fitness effects were quantified as s-scores, that is, a modified t-statistic on the deviation of the colony size of one mutant in one condition from the median colony size of the mutant across all conditions38,78. We reprocessed the data to exclude the following: (1) strains from the hypomorphic mutant collection and mutants that had ≥10 missing values for the conditions, reaching a final number of 3,904 mutants, and (2) environmental stresses (for example, different temperatures, pH, heavy metals, amino acids, dyes and alternative carbon sources), non-antibiotic drugs and drug combinations. Antibiotics with a narrow range of s-scores (no extreme s-scores, that is, <−6.9 or >3.9) were also excluded from the analysis (n = 7). This left us with 40 antibiotics that were further used in this study (Supplementary Table 1). For those antibiotics tested in multiple concentrations, the highest was selected.
Previously reported XR and CS interactions were collected from four studies. Lazar and colleagues17,18 measured XR and CS in E. coli BW25113 using 12 antibiotics where interactions were defined based on a difference of at least 10% in the growth of more than 50% evolved lineages compared with control lineages. Oz and colleagues19, and Imamovic and Sommer8 compared the MICs of evolved populations with the wild type to define XR and CS in E. coli MG1655 using 22 and 23 antibiotics, respectively. We kept the original definitions and assessments of XR and CS used in the respective studies. When integrating these datasets, interactions of overlapping antibiotic pairs were annotated as ‘XR and neutral’, ‘CS and neutral’, ‘XR and CS’ and ‘XR and CS and neutral’ if conflicting interactions were observed in different studies. Interactions with ‘XR and CS’ and ‘XR and CS and neutral’ annotations were removed (n = 6) and ‘XR and neutral’ and ‘CS and neutral’ were re-annotated as ‘XR’ and ‘CS’, respectively, because evolution experiments are prone to false negatives. Directionality was reduced (keeping drug 1–drug 2 but removing the reciprocal) by removing one pair (if XR/CS was bidirectional) or the ‘neutral pair’ (if the interaction was unidirectional). After the preprocessing steps, only conditions for which chemical genetics data were available were selected as the training set (n = 24), amounting to 111 neutral, 70 XR and 25 CS drug-pair relationships (Supplementary Table 3).
Assessment of correlation metrics
Given that the first attempts at combining chemical genetics profiles and XR/CS interactions found associations between the chemical genetics profile similarity and XR/CS17,18, we assessed several correlation methods from SciPy (v1.12.0)79 to compute various correlation coefficients between two drugs (drugs 1 and 2; Extended Data Fig. 1a). The correlation functions were applied to drug pairs with known interactions for which chemical genetics data are available. For each drug pair in this dataset, the correlation coefficient was computed using the four methods (Pearson, Spearman, Kendall’s tau and weighted tau). We plotted ROC curves to evaluate the performance of the computed correlation coefficients in distinguishing between interaction types (XR (n = 70) versus non-XR (n = 136) and CS (n = 25) versus non-CS (n = 181)). The correlation coefficients served as the predictor values and the interaction types (either XR or CS) were the true labels. The ROC AUC was computed for each correlation method (Extended Data Fig. 1a).
Feature generation and interpretation of decision trees
For each condition in the chemical genetics data, 3% extreme positive and negative s-scores were chosen after assessment of different cutoffs (Extended Data Fig. 1d). Six features were generated by antibiotics pairwise calculation: sum of positive concordant s-scores, sum of negative concordant s-scores, sum of discordant s-scores, count of positive concordant s-scores, count of negative concordant s-scores and count of discordant s-scores. Using these features, machine-learning algorithms (based on decision trees80) were used and models were trained to classify XR (n = 70) versus non-XR (n = 136) and CS (n = 25) versus non-CS (n = 181).
To address the class imbalance, the minority class was oversampled to match the size of the majority class. A search space for hyperparameters was defined for the decision tree classifier, including the function to measure the quality of a split, the maximum depth of the tree, the minimum number of samples required to split an internal node and the minimum number of samples required to be at a leaf node. A fivefold grid search cross-validation always excluding the test set from the training set, stratified to maintain the same proportion of the target class as the entire dataset, was used to find the best hyperparameters for the decision tree classifier based on the F1 score. The resulting classifier was trained and again evaluated on the balanced dataset using cross-validation. The best classifier according to the F1 score, precision, recall and ROC AUC was then fitted to the balanced dataset.
The trained decision tree classifier was graphed, showing the decision paths and splits. The tree visualization was limited to a depth of three for clarity (Extended Data Fig. 1c). We learned from decision tree classifiers that if the count of concordant negative s-scores was higher, the level of discordance was not important to classify interactions. The sum and count of concordant negative s-scores were found to be the most important features, followed by the sum of discordant s-scores. This information was used to generate the OCDM metric, described in detail in the following section. Classifier training, hyperparameter tuning and visualization were implemented using the scikit-learn package (v1.1.3)81.
Metric generation and interaction measurement
Among the correlation methods, six chemical genetics-derived features and their engineered combinations, we identified the OCDM as the best metric to separate statistically significantly XR, neutral and CS interactions (Fig. 2c). The OCDM metric is defined as the difference between the sum of concordant s-scores and the sum of discordant s-scores if the count of concordant s-scores (NC) is lower than the median count as shown below. Otherwise, OCDM is simply the sum of concordant s-scores.
where C represents concordant s-scores and D represents discordant s-scores. To identify optimal threshold determination (cutoffs) of OCDM, the false-positive (FPR) and true-positive (TPR) rates were used to calculate the true factor (TF = TPR − (1 − FPR) = sensitivity − specificity), which was computed for each threshold. This threshold represents the best trade-off between sensitivity (TPR) and specificity (1 − FPR), which are >105.159057 (to define XR) and <27.224792 (to define CS).
All data analyses were performed in Python (v3.9.17).
Bacterial strains and growth conditions
For all experiments, and unless otherwise specified, E. coli (strain BW25113) or single-gene knockouts in this strain60 were cultured in LB Lennox broth (tryptone 10 g l−1, yeast extract 5 g l−1 and sodium chloride 5 g l−1) at 37 °C and fully aerobically (850 rpm) or on agar (2%) plates (same medium and temperature).
MIC (IC90) determination
Overnight cultures of E. coli BW25113 were diluted to OD600mn = 0.001 and cultured with antibiotics (Supplementary Table 1) at eight concentrations in a twofold dilution gradient, in two technical replicates in microtiter plates (U-bottomed 96-well plates; Greiner Bio-One, 268200) at 37 °C with continuous shaking (850 rpm; orbital microplate shaking). The plates were sealed with breathable membrane (Breathe-Easy; Sigma-Aldrich, Z380059-1PAK) and the OD600nm was measured every 30 min for 24 h using the BioTek Gen5 (v3.02.2) and SoftMax Pro 7.1 software. The liquid handler Biomek FX (Beckman Coulter) was used to prepare plates. All MIC tests were performed in a total volume of 100 µl per well. Controls included ‘no cell + no drug’ controls to assess contamination, ‘no drug’ controls to assess maximal growth and ‘no cell’ controls to assess artefacts (OD600mn change) of the drugs alone or their interaction with medium components. The AUC was calculated using the simps function from SciPy (v1.12.0)79 and divided by the no drug control. Across the study, the MICs were defined as the IC90, which was calculated using the drc (v0.5.8) package in R (v.4.1.2)82.
Experimental evolution and XR/CS measurements
Overnight cultures of wild-type E. coli were diluted 1:1,000 and exposed to eight concentrations—from 0.5× IC90 to 64× IC90—of 23 antibiotics in 12 lineages using the same volumes and plates as for MIC determination. Every 24 h the lineages growing in the highest concentration (OD600nm > 0.3) were back-diluted to OD600nm = 0.01 and the volume needed to reach a final dilution of 1:1,000 (3–10 µl) was transferred to the next plate with the same concentration gradients. Once the evolution experiment was completed (five passages for a total of five days; approximately 50 generations in total), the lineages were tested for antibiotic susceptibility for 70 of the 634 predicted interactions (11%; 30 novel XR, eight known XR, 25 novel CS, two known CS, four novel neutral and one known neutral interaction; Fig. 3b–d and Source Data Fig. 3). The IC90 values were determined as in ‘MIC determination’ (12 lineages or populations × 140 combinations (70 unique drug pairs) × 2 technical replicates = 3,360 IC90 values; Source Data Fig. 3c–e). Changes in IC90 were compared with the ancestor strain. Interactions were defined as XR or CS if log2(fold change) > +1 or −1, respectively. For 14 drug pairs, we performed five more passages (total of ten passages; approximately 100 generations) and measured the changes in IC90 again (Extended Data Fig. 2e). In the case of the azithromycin–tetracycline pair, we tracked changes both in tetracycline resistance and azithromycin susceptibilities across multiple generations.
Whole-genome sequencing and analysis
A clone from the wild type and from populations of 12 lineages from days 3, 5 and 7 were sequenced to determine mutations responsible for the given phenotype. Genomic DNA was extracted using a Macherey Nagel DNA extraction kit and sequenced using single-end Illumina NextSeq 2000 (P1; length of 122 bp). Mutations were identified by mapping sequences to the reference genome from the NCBI database (E. coli BW25113 strain K-12 chromosome; GCF_000750555.1)83 using Breseq84 with the following parameters: -p -l 80 -j 8 -b 5 -m 30. Mutations present in the wild-type clone compared with the NCBI reference genome were eliminated to only identify mutations that are associated with resistance/sensitivity.
P1 transduction
Single colonies of E. coli wild type (BW25113) and the corresponding Keio mutants60 were used for P1 transduction. P1 lysate preparation and transduction were performed as previously described85. We confirmed the transduction success with colony PCR.
Tetracycline fluorescence assay
Wild-type E. coli and waaF- waaD- and hldE-knockout mutants were cultured in 5 ml LB with continuous shaking at 37 °C until they reached an OD600nm of 0.5. Aliquots (1 ml) of each culture were centrifuged at 3,500 rpm (1,300g) for 10 min and the supernatants were discarded. The pellets were washed three times with 0.5 ml of 137 mM PBS, resuspended in 50 µl of 137 mM PBS and transferred to black-walled, clear- and flat-bottomed 96-well plates (Greiner Bio-One, 655096) containing three concentrations of twofold serially diluted tetracycline (highest final concentration, 16 µg ml−1; final volume, 100 µl per well). Both the OD600nm and fluorescence (excitation λ, 405 nm; emission λ, 535 nm) were measured with an Infinite M1000 PRO plate reader (Tecan i-control (v1.10)) for 15 min, with readings taken every minute. Three to six biological replicates were conducted for each experiment.
Experimental evolution against antibiotic combinations
The IC90 values for individual antibiotics (n = 8) and drug combinations at a 1:1 IC90 ratio (n = 7) were measured as in ‘MIC determination’. The evolution experiment was carried out in the same way as described in ‘Experimental evolution and XR/CS measurements’ with the following changes: the initial wild-type culture was exposed to 11 concentrations—from 0.125× IC90 to 128× IC90—of eight single antibiotics and seven antibiotic combinations for seven lineages. At the end of the experiment (seven passages for a total of seven days; approximately 70 generations), the IC90 values of drugs 1 and 2 were measured in drug 1-, drug 2- and drug 1 + 2-resistant lineages as described in ‘MIC measurements’. To compare the evolution of resistance to single drugs versus drug combinations, evolvability indices were calculated using the average of the log2-transformed IC90 ratios of two drugs for each possible pair (2,401 values per antibiotic combination) as:
where IC90(drug 1)drug1+2 corresponds to the IC90 of drug 1 for the lineage evolved against the drug 1 + 2 combination. We calculated evolvability indices using the modified equation, an average of the log2-transformed IC90 ratios of two drugs, different from previously defined (equation (3))21 as it assesses the effects of combining drugs on resistance to each drug separately.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All supplementary data are provided in Supplementary Tables 1–3. A reference genome from the NCBI database (E. coli BW25113 strain K-12 chromosome, GCF_000750555.1) was used. Raw reads of sequenced samples (file names describe samples) are available via Zenodo at https://doi.org/10.5281/zenodo.10572857 (ref. 86). All data are included in the Shiny app at https://shiny-portal.embl.de/shinyapps/app/21_xrcs. Source data are provided with this paper.
References
Murray, C. J. L. et al. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet 399, 629–655 (2022).
Theuretzbacher, U. et al. Analysis of the clinical antibacterial and antituberculosis pipeline. Lancet Infect. Dis. 19, e40–e50 (2019).
Butler, M. S., Henderson, I. R., Capon, R. J. & Blaskovich, M. A. T. Antibiotics in the clinical pipeline as of December 2022. J. Antibiot. 76, 431–473 (2023).
Szybalski, W. & Bryson, V. Genetic studies on microbial cross-resistance to toxic agents I: cross resistance of Escherichia coli to fifteen antibiotics. J. Bacteriol. 64, 489–499 (1952).
Beutner, E. H., Doyle, W. M. & Evander, L. C. Collateral susceptibility of isoniazid-resistant tubercle bacilli to nitrofurans. Am. Rev. Respiratory Dis. 88, 712–715 (1963).
Baym, M., Stone, L. K. & Kishony, R. Multidrug evolutionary strategies to reverse antibiotic resistance. Science 351, aad3292 (2016).
Roemhild, R., Linkevicius, M. & Andersson, D. I. Molecular mechanisms of collateral sensitivity to the antibiotic nitrofurantoin. PLoS Biol. 18, e3000612 (2020).
Imamovic, L. & Sommer, M. O. A. Use of collateral sensitivity networks to design drug cycling protocols that avoid resistance development. Sci. Transl. Med. 5, 204ra132 (2013).
Kim, S., Lieberman, T. D. & Kishony, R. Alternating antibiotic treatments constrain evolutionary paths to multidrug resistance. Proc. Natl Acad. Sci. USA 111, 14494–14499 (2014).
Barbosa, C., Beardmore, R., Schulenburg, H. & Jansen, G. Antibiotic combination efficacy (ACE) networks for a Pseudomonas aeruginosa model. PLoS Biol. 16, e2004356 (2018).
Roemhild, R. et al. Cellular hysteresis as a principle to maximize the efficacy of antibiotic therapy. Proc. Natl Acad. Sci. USA 115, 9767–9772 (2018).
Hernando-Amado, S., Sanz-García, F. & Martínez, J. L. Rapid and robust evolution of collateral sensitivity in Pseudomonas aeruginosa antibiotic-resistant mutants. Sci. Adv. 6, eaba5493 (2020).
Aulin, L. B. S., Liakopoulos, A., van der Graaf, P. H., Rozen, D. E. & van Hasselt, J. G. C. Design principles of collateral sensitivity-based dosing strategies. Nat. Commun. 12, 5691 (2021).
Jahn, L. J. et al. Compatibility of evolutionary responses to constituent antibiotics drive resistance evolution to drug pairs. Mol. Biol. Evol. 38, 2057–2069 (2021).
Hernando-Amado, S., Laborda, P. & Martínez, J. L. Tackling antibiotic resistance by inducing transient and robust collateral sensitivity. Nat. Commun. 14, 1723 (2023).
Barbosa, C., Römhild, R., Rosenstiel, P. & Schulenburg, H. Evolutionary stability of collateral sensitivity to antibiotics in the model pathogen Pseudomonas aeruginosa. eLife 8, e51481 (2019).
Lázár, V. et al. Bacterial evolution of antibiotic hypersensitivity. Mol. Syst. Biol. 9, 700 (2013).
Lázár, V. et al. Genome-wide analysis captures the determinants of the antibiotic cross-resistance interaction network. Nat. Commun. 5, 4352 (2014).
Oz, T. et al. Strength of selection pressure is an important parameter contributing to the complexity of antibiotic resistance evolution. Mol. Biol. Evol. 31, 2387–2401 (2014).
Arcangioli, M.-A., Leroy-Setrin, S., Martel, J.-L. & Chaslus-Dancla, E. Evolution of chloramphenicol resistance, with emergence of cross-resistance to florfenicol, in bovine Salmonella Typhimurium strains implicates definitive phage type (DT) 104. J. Med. Microbiol. 49, 103–110 (2000).
Rodriguez de Evgrafov, M., Gumpert, H., Munck, C., Thomsen, T. T. & Sommer, M. O. A. Collateral resistance and sensitivity modulate evolution of high-level resistance to drug combination treatment in Staphylococcus aureus. Mol. Biol. Evol. 32, 1175–1185 (2015).
Barbosa, C. et al. Alternative evolutionary paths to bacterial antibiotic resistance cause distinct collateral effects. Mol. Biol. Evol. 34, 2229–2244 (2017).
Imamovic, L. et al. Drug-driven phenotypic convergence supports rational treatment strategies of chronic infections. Cell 172, 121–134 (2018).
Laborda, P., Martínez, J. L. & Hernando-Amado, S. Convergent phenotypic evolution towards fosfomycin collateral sensitivity of Pseudomonas aeruginosa antibiotic-resistant mutants. Microb. Biotechnol. 15, 613–629 (2022).
Hernando-Amado, S., Laborda, P., Valverde, J. R. & Martínez, J. L. Mutational background influences P. aeruginosa ciprofloxacin resistance evolution but preserves collateral sensitivity robustness. Proc. Natl Acad. Sci. USA 119, e2109370119 (2022).
Liu, D. Y. et al. Collateral sensitivity profiling in drug-resistant Escherichia coli identifies natural products suppressing cephalosporin resistance. Nat. Commun. 14, 1976 (2023).
Suzuki, S., Horinouchi, T. & Furusawa, C. Prediction of antibiotic resistance by gene expression profiles. Nat. Commun. 5, 5792 (2014).
Horinouchi, T. et al. Prediction of cross-resistance and collateral sensitivity by gene expression profiles and genomic mutations. Sci. Rep. 7, 14009 (2017).
Brochado, A. R. & Typas, A. High-throughput approaches to understanding gene function and mapping network architecture in bacteria. Curr. Opin. Microbiol. 16, 199–206 (2013).
Cacace, E., Kritikos, G. & Typas, A. Chemical genetics in drug discovery. Curr. Opin. Syst. Biol. 4, 35–42 (2017).
Nichols, R. J. et al. Phenotypic landscape of a bacterial cell. Cell 144, 143–156 (2011).
Ezraty, B. et al. Fe-S cluster biosynthesis controls uptake of aminoglycosides in a ROS-less death pathway. Science 340, 1583–1587 (2013).
Chandrasekaran, S. et al. Chemogenomics and orthology-based design of antibiotic combination therapies. Mol. Syst. Biol. 12, 872 (2016).
Shiver, A. L. et al. A chemical–genomic screen of neglected antibiotics reveals illicit transport of kasugamycin and blasticidin S. PLoS Genet. 12, e1006124 (2016).
Brochado, A. R. et al. Species-specific activity of antibacterial drug combinations. Nature 559, 259–263 (2018).
Silvis, M. R. et al. Morphological and transcriptional responses to CRISPRi knockdown of essential genes in Escherichia coli. mBio 12, e0256121 (2021).
Kintses, B. et al. Chemical-genetic profiling reveals limited cross-resistance between antimicrobial peptides with different modes of action. Nat. Commun. 10, 5731 (2019).
Collins, S. R., Schuldiner, M., Krogan, N. J. & Weissman, J. S. A strategy for extracting and analyzing large-scale quantitative epistatic interaction data. Genome Biol. 7, R63 (2006).
Pál, C., Papp, B. & Lázár, V. Collateral sensitivity of antibiotic-resistant microbes. Trends Microbiol. 23, 401–407 (2015).
Møller, A. K. et al. An Escherichia coli MG1655 lipopolysaccharide deep-rough core mutant grows and survives in mouse cecal mucus but fails to colonize the mouse large intestine. Infect. Immun. 71, 2142–2152 (2003).
Nobre, T. M. et al. Modification of Salmonella lipopolysaccharides prevents the outer membrane penetration of novobiocin. Biophys. J. 109, 2537–2545 (2015).
Mateus, A. et al. The functional proteome landscape of Escherichia coli. Nature 588, 473–478 (2020).
Mortimer, P. G. & Piddock, L. J. The accumulation of five antibacterial agents in porin-deficient mutants of Escherichia coli. J. Antimicrob. Chemother. 32, 195–213 (1993).
Hu, M., Nandi, S., Davies, C. & Nicholas, R. A. High-level chromosomally mediated tetracycline resistance in Neisseria gonorrhoeae results from a point mutation in the rpsJ gene encoding ribosomal protein S10 in combination with the mtrR and penB resistance determinants. Antimicrob. Agents Chemother. 49, 4327–4334 (2005).
Grkovic, S., Brown, M. H. & Skurray, R. A. Regulation of bacterial drug export systems. Microbiol. Mol. Biol. Rev. 66, 671–701 (2002).
Beggs, G. A., Brennan, R. G. & Arshad, M. MarR family proteins are important regulators of clinically relevant antibiotic resistance. Protein Sci. 29, 647–653 (2020).
Price, M. N. et al. Mutant phenotypes for thousands of bacterial genes of unknown function. Nature 557, 503–509 (2018).
Cohen, S. P., McMurry, L. M., Hooper, D. C., Wolfson, J. S. & Levy, S. B. Cross-resistance to fluoroquinolones in multiple-antibiotic-resistant (Mar) Escherichia coli selected by tetracycline or chloramphenicol: decreased drug accumulation associated with membrane changes in addition to OmpF reduction. Antimicrob. Agents Chemother. 33, 1318–1325 (1989).
Thanassi, D. G., Suh, G. S. & Nikaido, H. Role of outer membrane barrier in efflux-mediated tetracycline resistance of Escherichia coli. J. Bacteriol. 177, 998–1007 (1995).
Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656 (2003).
Yen, P. & Papin, J. A. History of antibiotic adaptation influences microbial evolutionary dynamics during subsequent treatment. PLoS Biol. 15, e2001586 (2017).
Podnecky, N. L. et al. Conserved collateral antibiotic susceptibility networks in diverse clinical strains of Escherichia coli. Nat. Commun. 9, 3673 (2018).
Peters, J. M. et al. A comprehensive, CRISPR-based functional analysis of essential genes in bacteria. Cell 165, 1493–1506 (2016).
Johnson, E. O. et al. Large-scale chemical–genetics yields new M. tuberculosis inhibitor classes. Nature 571, 72–78 (2019).
Liu, H. et al. Functional genetics of human gut commensal Bacteroides thetaiotaomicron reveals metabolic requirements for growth across environments. Cell Rep. 34, 108789 (2021).
Shiver, A. L. et al. A mutant fitness compendium in bifidobacteria reveals molecular determinants of colonization and host–microbe interactions. Preprint at bioRxiv https://doi.org/10.1101/2023.08.29.555234 (2023).
Rosconi, F. et al. A bacterial pan-genome makes gene essentiality strain-dependent and evolvable. Nat. Microbiol. 7, 1580–1592 (2022).
Rousset, F. et al. The impact of genetic diversity on gene essentiality within the Escherichia coli species. Nat. Microbiol. 6, 301–312 (2021).
Voogdt, C. G. P. et al. Randomly barcoded transposon mutant libraries for gut commensals II: applying libraries for functional genetics. Cell Rep. 43, 113519 (2023).
Baba, T. et al. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006.0008 (2006).
Porwollik, S. et al. Defined single-gene and multi-gene deletion mutant collections in Salmonella enterica sv Typhimurium. PLoS ONE 9, e99820 (2014).
Koo, B.-M. et al. Construction and analysis of two genome-scale deletion libraries for Bacillus subtilis. Cell Syst. 4, 291–305 (2017).
Tripathi, S. et al. Randomly barcoded transposon mutant libraries for gut commensals I: strategies for efficient library construction. Cell Rep. 43, 113517 (2023).
de Bakker, V., Liu, X., Bravo, A. M. & Veening, J.-W. CRISPRi-seq for genome-wide fitness quantification in bacteria. Nat. Protoc. 17, 252–281 (2022).
Costanzo, M. et al. Environmental robustness of the global yeast genetic interaction network. Science 372, eabf8424 (2021).
Batra, A. et al. High potency of sequential therapy with only β-lactam antibiotics. eLife 10, e68876 (2021).
Rosenkilde, C. E. H. et al. Collateral sensitivity constrains resistance evolution of the CTX-M-15 β-lactamase. Nat. Commun. 10, 618 (2019).
Nichol, D. et al. Antibiotic collateral sensitivity is contingent on the repeatability of evolution. Nat. Commun. 10, 334 (2019).
Apjok, G. et al. Limited evolutionary conservation of the phenotypic effects of antibiotic resistance mutations. Mol. Biol. Evol. 36, 1601–1611 (2019).
Wright, G. D. Antibiotic adjuvants: rescuing antibiotics from resistance. Trends Microbiol. 24, 862–871 (2016).
Maier, L. et al. Extensive impact of non-antibiotic drugs on human gut bacteria. Nature 555, 623–628 (2018).
Tyers, M. & Wright, G. D. Drug combinations: a strategy to extend the life of antibiotics in the 21st century. Nat. Rev. Microbiol. 17, 141–155 (2019).
Allen, R. C., Pfrunder-Cardozo, K. R. & Hall, A. R. Collateral sensitivity interactions between antibiotics depend on local abiotic conditions. mSystems 6, e0105521 (2021).
Santos-Lopez, A., Marshall, C. W., Scribner, M. R., Snyder, D. J. & Cooper, V. S. Evolutionary pathways to antibiotic resistance are dependent upon environmental structure and bacterial lifestyle. eLife 8, e47612 (2019).
Björkman, J., Nagaev, I., Berg, O. G., Hughes, D. & Andersson, D. I. Effects of environment on compensatory mutations to ameliorate costs of antibiotic resistance. Science 287, 1479–1482 (2000).
Cacace, E. et al. Systematic analysis of drug combinations against Gram-positive bacteria. Nat. Microbiol. 8, 2196–2212 (2023).
Lázár, V., Snitser, O., Barkan, D. & Kishony, R. Antibiotic combinations reduce Staphylococcus aureus clearance. Nature 610, 540–546 (2022).
Kritikos, G. et al. A tool named Iris for versatile high-throughput phenotyping in microorganisms. Nat. Microbiol. 2, 17014 (2017).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Breiman, L., Friedman, J., Olshen, R. A. & Stone, C. J. Classification and Regression Trees (Routledge, 2017).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Ritz, C., Baty, F., Streibig, J. C. & Gerhard, D. Dose–response analysis using R. PLoS ONE 10, e0146021 (2015).
Sayers, E. W. et al. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 50, D20–D26 (2021).
Deatherage, D. E. & Barrick, J. E. Identification of mutations in laboratory evolved microbes from next-generation sequencing data using breseq. Methods Mol. Biol. 1151, 165–188 (2014).
Thomason, L. C., Costantino, N. & Court, D. L. E. coli genome manipulation by P1 transduction. Curr. Protoc. Mol. Biol. 79, 1.17.1–1.17.8 (2007).
Sakenova, N. et al. Systematic mapping of antibiotic cross-resistance and collateral sensitivity with chemical genetics. Zenodo https://doi.org/10.5281/zenodo.10572857 (2024).
Acknowledgements
We thank M. Galardini, A. Koumoutsi and the EMBL Gene Core for help with sequencing experiments; A. Koumoutsi, L. Y. Yong and S. Bassler for experimental advice as well as the Typas laboratory, especially K. Mitosch, for fruitful discussions. This work was supported by the ERC consolidator grant uCARE (ID 819454) and EMBL internal funding to A.T.
Funding
Open access funding provided by European Molecular Biology Laboratory (EMBL).
Author information
Authors and Affiliations
Contributions
A.T. and N.S. conceived and designed the study. A.T., C.V.G., E.C., P.B. and J.M. supervised the project. All scripts were written by N.S., with advice on data preprocessing from F.H., machine learning from A.O. and MIC determination from V.V. All experiments were carried out by N.S. with advice from E.C. and C.V.G. Figures were designed and plotted by N.S. with input from E.C., A.O., C.V.G. and A.T. N.S. and A.T. wrote the manuscript with input from all authors. All authors approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks the anonymous reviewers for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Performance of different metrics and models in capturing XR and CS antibiotic interactions from chemical genetics data.
a, Receiver operating characteristic (ROC) curves for classification of XR (positive class) vs non-XR (negative class), and CS (positive class) vs non-CS (negative class), using simple linear and non-linear correlation metrics. AUC is the area under the curve. b, The performance of the decision tree model on balanced classes shows that both XR and CS interactions can be well classified. c, Decision tree with classes CS (class 1) versus the rest (class 0), where a maximum depth of 3 is shown for visualization, illustrates the hierarchy of decisions to discriminate classes. Each node in the tree represents a decision point based on the value of a particular feature, and branches represent the outcome of the decision. The root node divides the data based on the ‘concordant_negative_w’ feature, which is the sum of s-scores (as weights) of hits on the negative concordant site of a scatterplot. The tree branches out to ‘discordant_w’ feature, which is the sum of s-scores (as weights) of hits on the discordant site of a scatterplot, while ‘discordant_w_m’ is the sum of products of s-scores (as weights) of hits on the discordant site of a scatterplot. d, P values from a paired Mann–Whitney U-test (two-sided) are depicted across quantile cutoffs for extreme s-scores to differentiate XR/CS/neutral interactions based on OCDM values. Q3 and Q97 perform the best. e, Confusion matrix of results based on Q3 and Q97. Most interactions inferred as non-XR/non-CS were previously reported neutral. For more information, see also Extended Data Fig. 2a-c.
Extended Data Fig. 2 Chemical genetics metric captures well prior information and can be used to reclassify a subset of prior interactions.
a,b, Comparison of previously reported XR (a) and CS (b) interactions with our inferences based on our chemical genetics metric (OCDM) show an agreement of 67–68% for CS (n=17) and XR (n=47) - 10 such interactions were validated experimentally during our benchmarking (Fig. 3b,d). The rest is inferred as neutral or the opposite interaction by OCDM, including seven interactions (4 CS, 2 neutral & 1 XR) that we experimentally validated that OCDM inference was correct (Fig. 3b,d). c, In contrast to CS or XS, there is less agreement for neutral interactions with previous studies. This is consistent with the high false negative rates when comparing prior studies between them (Fig. 2a). The majority of previously reported neutral interactions (76.6%, n=85) are inferred as CS/XR by chemical genetics. 11/13 we included in the benchmarking set were confirmed as inferred by OCDM. The other two were inferred CS, but although most lineages exhibited CS, a single lineage exhibited XR, and hence called XR (Fig. 3b–d). d, New XR, neutral, and CS pairs inferred by chemical genetics and the OCDM cutoff are 2.8- and 6.4-fold more than currently known XR and CS antibiotic interactions in E. coli, after reclassifying interactions (n = 116) we infer differently than previously reported. The plot includes known interactions for which chemical genetics data is not available. e, Resistance against 12 antibiotics was evolved again for up to ~100 generations in 12 lineages. The MIC of evolved populations was measured at ~50 and ~100 generations for the same lineages in different antibiotics, allowing us to assess XR/CS for 17 drug pairs in both directions. Data are represented and drug pairs are numbered as in Fig. 3b,d. All inferred interactions were validated at both ~50 and ~100 generations. The length of experimental evolution affected the XR/CS of individual lineages and to a lower degree the cumulative call of the drug pair.
Extended Data Fig. 3 Chemical genetics can uncover the biological processes that drive interactions between antibiotic classes.
a, Clustered heatmap of discordant mutants that are part of CS interactions between sulfonamides and macrolides (blue) or beta-lactams (green). Genes in bold are involved in LPS or nucleotide biosynthesis. b, Clustered heatmap of concordant mutants that are part of XR interactions between tetracyclines (violet), macrolides (blue), and other protein synthesis inhibitors. Genes in bold regulate or are part of the major efflux pump in E. coli (AcrAB-TolC).
Extended Data Fig. 4 Chemical genetics can infer mechanisms and monochromaticity of XR and CS drug interactions.
a, Scatter plot of chemical genetic profiles of the E. coli deletion library in tobramycin and nalidixic acid31. Mutants with concordant (XR-related) and discordant (CS-related) profiles are highlighted. Dots in grey represent mutants that do not have s-scores within the 3% extreme values for both drugs. The underlined knockout mutants are known causal genes of this CS interaction17,19. b, Chemical genetic profiles for novobiocin and cefoxitin, presented as in a. Underlined knockout mutants indicate that the changes in polarity of the lipopolysaccharide (LPS) core can drive resistance to cefoxitin while providing sensitivity to the large and non-polar novobiocin. c, Non-monochromatic XR interactions (n=11) have higher absolute discordance scores than their monochromatic counterparts (n=27) (two-sided Mann–Whitney U-test; P = 3.758e-07) - monochromaticity was defined in the validation experiment. This means that chemical genetics can infer the monochromaticity of XR interactions. The box boundaries represent the first and third quartiles, with the median indicated. The whiskers extend to the furthest data points within 1.5 times the interquartile range (IQR). d, The highest discordance score of -133.8481 based on the 11 non-monochromatic XR interactions from c was used to separate the remaining inferred XR interactions (excluding the 38 validated) into monochromatic (n=225) or non-monochromatic (n=168). e–g. Scatter plots of chemical genetic profiles of the E. coli deletion library31 for examples of other pairs of drugs with both high concordance and discordance (in addition to azithromycin and tetracycline shown in Fig. 5b). As the azithromycin-tetracycline pair, those are expected to be non-monochromatic. Data are depicted as in a,b. For all data, see the relevant Shiny app at https://shiny-portal.embl.de/shinyapps/app/21_xrcs.
Extended Data Fig. 6 CS antibiotic combinations constrain resistance evolution to one or both compounds.
a, log2 transformed evolvability Index as originally proposed in the literature (equation (3), Methods) confirms observations made using the slightly modified Evolvability Index (equation (2), Methods) – Fig. 6b. Red dots on the violin plots represent the median. The box boundaries represent the first and third quartiles, with the median indicated. The whiskers extend to the furthest data points within 1.5 times the interquartile range (IQR). b, The log2 of MIC (IC90) of the evolved population in both drugs compared evolved population of the drug itself is used to identify whether and how well combining drugs reduces resistance to each drug compared to single-drug treatments. Reciprocal CS drug pairs do this efficiently. The red dashed line shows the no-effect when combining drugs does not change resistance evolution to single drug treatments. The bars in the violin plots represent the distributions of log2 MIC ratios for each antibiotic combination.
Supplementary information
Supplementary Table 1
Chemical genetics data used in this study31.
Supplementary Table 2
Predictions of XR, neutral and CS using OCDM with gene sets (discordant mutants if CS and concordant mutants if XR; if neutral, none).
Supplementary Table 3
Drugs used in this study.
Source data
Source Data Fig. 2
Source data table.
Source Data Fig. 3
Source data table.
Source Data Fig. 4
Source data table.
Source Data Fig. 5
Source data table.
Source Data Fig. 6
Source data table.
Source Data Extended Data Fig. 1
Source data table.
Source Data Extended Data Fig. 2
Source data table.
Source Data Extended Data Fig. 3
Source data table.
Source Data Extended Data Fig. 4
Source data table.
Source Data Extended Data Fig. 6
Source data table.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sakenova, N., Cacace, E., Orakov, A. et al. Systematic mapping of antibiotic cross-resistance and collateral sensitivity with chemical genetics. Nat Microbiol 10, 202–216 (2025). https://doi.org/10.1038/s41564-024-01857-w
Received:
Accepted:
Published:
Issue date:
DOI: https://doi.org/10.1038/s41564-024-01857-w
This article is cited by
-
Bioenergetic stress potentiates antimicrobial resistance and persistence
Nature Communications (2025)
-
Cross resistance emergence to polymyxins in Acinetobacter baumannii exposed in vitro to an antimicrobial peptide
npj Antimicrobials and Resistance (2025)
-
Evolutionary drivers of divergent collateral sensitivity responses during antibiotic therapy
Nature Ecology & Evolution (2025)
-
Antimicrobial peptides: structure, functions and translational applications
Nature Reviews Microbiology (2025)