Abstract
Cell-autonomous immunity prevents intracellular pathogen growth through mechanisms such as ubiquitination and proteasomal targeting of bacteria for degradation. However, how the proteasome eradicates ubiquitinated bacteria has remained unclear. Here we show that host AAA-ATPase, VCP/p97, associates with diverse cytosol-exposed ubiquitinated bacteria (Streptococcus pneumoniae, Salmonella enterica serovar Typhimurium, Streptococcus pyogenes) and requires the ATPase activity in its D2 domain to reduce intracellular bacterial loads. Combining optical trap approaches along with molecular dynamic simulations, in vitro reconstitution and immunogold transmission electron microscopy, we demonstrate that p97 applies mechanical force to extract ubiquitinated surface proteins, BgaA and PspA, from S. pneumoniae cell membranes. This causes extensive membrane lysis and release of cytosolic content, thereby killing the pathogen. Further, p97 also controls S. pneumoniae proliferation in mice, ultimately protecting from fatal sepsis. Overall, we discovered a distinct innate antimicrobial function of p97 that can protect the host against lethal bacterial infections.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$32.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on SpringerLink
- Instant access to the full article PDF.
USD 39.95
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the Article and its Supplementary Information. Reagents pertaining to the Article are available from the corresponding author. Source data are provided with this paper.
References
Randow, F., MacMicking, J. D. & James, L. C. Cellular self-defense: how cell-autonomous immunity protects against pathogens. Science 340, 701–706 (2013).
Asmat, T. M., Agarwal, V., Saleh, M. & Hammerschmidt, S. Endocytosis of Streptococcus pneumoniae via the polymeric immunoglobulin receptor of epithelial cells relies on clathrin and caveolin dependent mechanisms. Int. J. Med. Microbiol. 304, 1233–1246 (2014).
Lilic, M. et al. Salmonella SipA polymerizes actin by stapling filaments with nonglobular protein arms. Science 301, 1918–1921 (2003).
Kaufmann, S. H. Intracellular pathogens: living in an extreme environment. Immunol. Rev. 240, 5–10 (2011).
Ray, K., Marteyn, B., Sansonetti, P. J. & Tang, C. M. Life on the inside: the intracellular lifestyle of cytosolic bacteria. Nat. Rev. Microbiol. 7, 333–340 (2009).
Badgujar, D. C. et al. Structural insights into loss of function of a pore forming toxin and its role in pneumococcal adaptation to an intracellular lifestyle. PLoS Pathog. 16, e1009016 (2020).
Surve, M. V. et al. Heterogeneity in pneumolysin expression governs the fate of Streptococcus pneumoniae during blood–brain barrier trafficking. PLoS Pathog. 14, e1007168 (2018).
Ellison, C. J., Kukulski, W., Boyle, K. B., Munro, S. & Randow, F. Transbilayer movement of sphingomyelin precedes catastrophic breakage of enterobacteria-containing vacuoles. Curr. Biol. 30, 2974–2983.e6 (2020).
Paz, I. et al. Galectin-3, a marker for vacuole lysis by invasive pathogens. Cell. Microbiol. 12, 530–544 (2010).
Feng, S. et al. Pathogen-selective killing by guanylate-binding proteins as a molecular mechanism leading to inflammasome signaling. Nat. Commun. 13, 4395 (2022).
Kutsch, M. et al. Direct binding of polymeric GBP1 to LPS disrupts bacterial cell envelope functions. EMBO J. 39, e104926 (2020).
Gaudet, R. G. et al. A human apolipoprotein L with detergent-like activity kills intracellular pathogens. Science 373, eabf8113 (2021).
Apte, S. et al. An innate pathogen sensing strategy involving ubiquitination of bacterial surface proteins. Sci. Adv. 9, eade1851 (2023).
Man, S. M. et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nat. Immunol. 16, 467–475 (2015).
Meunier, E. et al. Guanylate-binding proteins promote activation of the AIM2 inflammasome during infection with Francisella novicida. Nat. Immunol. 16, 476–484 (2015).
Buijze, H. et al. Human GBP1 is involved in the repair of damaged phagosomes/endolysosomes. Int. J. Mol. Sci. 24, 9701 (2023).
Deol, K. K., Lorenz, S. & Strieter, E. R. Enzymatic logic of ubiquitin chain assembly. Front. Physiol. 10, 835 (2019).
Dickinson, M. S. et al. LPS-aggregating proteins GBP1 and GBP2 are each sufficient to enhance caspase-4 activation both in cellulo and in vitro. Proc. Natl Acad. Sci. USA 120, e2216028120 (2023).
Perrin, A. J., Jiang, X., Birmingham, C. L., So, N. S. & Brumell, J. H. Recognition of bacteria in the cytosol of mammalian cells by the ubiquitin system. Curr. Biol. 14, 806–811 (2004).
Bhutda, S. et al. Differential ubiquitination as an effective strategy employed by the blood–brain barrier for prevention of bacterial transcytosis. J. Bacteriol. 204, e0045621 (2022).
Franco, L. H. et al. The ubiquitin ligase Smurf1 functions in selective autophagy of Mycobacterium tuberculosis and anti-tuberculous host defense. Cell Host Microbe 22, 421–423 (2017).
Yamada, A., Hikichi, M., Nozawa, T. & Nakagawa, I. FBXO2/SCF ubiquitin ligase complex directs xenophagy through recognizing bacterial surface glycan. EMBO Rep. 22, e52584 (2021).
Iovino, F., Gradstedt, H. & Bijlsma, J. J. The proteasome–ubiquitin system is required for efficient killing of intracellular Streptococcus pneumoniae by brain endothelial cells. mBio 5, e00984–00914 (2014).
Nickell, S. et al. Insights into the molecular architecture of the 26S proteasome. Proc. Natl Acad. Sci. USA 106, 11943–11947 (2009).
Xie, Y. Structure, assembly and homeostatic regulation of the 26S proteasome. J. Mol. Cell. Biol. 2, 308–317 (2010).
van den Boom, J. & Meyer, H. VCP/p97-mediated unfolding as a principle in protein homeostasis and signaling. Mol. Cell 69, 182–194 (2018).
DeLaBarre, B., Christianson, J. C., Kopito, R. R. & Brunger, A. T. Central pore residues mediate the p97/VCP activity required for ERAD. Mol. Cell 22, 451–462 (2006).
Noi, K. et al. High-speed atomic force microscopic observation of ATP-dependent rotation of the AAA+ chaperone p97. Structure 21, 1992–2002 (2013).
Khan, Y. A., White, K. I. & Brunger, A. T. The AAA+ superfamily: a review of the structural and mechanistic principles of these molecular machines. Crit. Rev. Biochem. Mol. Biol. 57, 156–187 (2022).
Chou, T. F. et al. Reversible inhibitor of p97, DBeQ, impairs both ubiquitin-dependent and autophagic protein clearance pathways. Proc. Natl Acad. Sci. USA 108, 4834–4839 (2011).
Magnaghi, P. et al. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nat. Chem. Biol. 9, 548–556 (2013).
Hao, Q. et al. A non-canonical role of the p97 complex in RIG-I antiviral signaling. EMBO J. 34, 2903–2920 (2015).
Dalal, S., Rosser, M. F., Cyr, D. M. & Hanson, P. I. Distinct roles for the AAA ATPases NSF and p97 in the secretory pathway. Mol. Biol. Cell 15, 637–648 (2004).
Zhang, X. et al. Altered cofactor regulation with disease-associated p97/VCP mutations. Proc. Natl Acad. Sci. USA 112, E1705–E1714 (2015).
Meyer, H. & Weihl, C. C. The VCP/p97 system at a glance: connecting cellular function to disease pathogenesis. J. Cell Sci. 127, 3877–3883 (2014).
Polajnar, M., Dietz, M. S., Heilemann, M. & Behrends, C. Expanding the host cell ubiquitylation machinery targeting cytosolic Salmonella. EMBO Rep. 18, 1572–1585 (2017).
Otten, E. G. et al. Ubiquitylation of lipopolysaccharide by RNF213 during bacterial infection. Nature 594, 111–116 (2021).
Hanzelmann, P. & Schindelin, H. The interplay of cofactor interactions and post-translational modifications in the regulation of the AAA+ ATPase p97. Front. Mol. Biosci. 4, 21 (2017).
Kubori, T. & Galan, J. E. Temporal regulation of Salmonella virulence effector function by proteasome-dependent protein degradation. Cell 115, 333–342 (2003).
Humphreys, D., Hume, P. J. & Koronakis, V. The Salmonella effector SptP dephosphorylates host AAA+ ATPase VCP to promote development of its intracellular replicative niche. Cell Host Microbe 5, 225–233 (2009).
Vonaesch, P. et al. The Salmonella Typhimurium effector protein SopE transiently localizes to the early SCV and contributes to intracellular replication. Cell. Microbiol. 16, 1723–1735 (2014).
Barak, P., Rai, A., Rai, P. & Mallik, R. Quantitative optical trapping on single organelles in cell extract. Nat. Methods 10, 68–70 (2013).
Ercoli, G. et al. Intracellular replication of Streptococcus pneumoniae inside splenic macrophages serves as a reservoir for septicaemia. Nat. Microbiol. 3, 600–610 (2018).
Hoffman, D. et al. A non-classical monocyte-derived macrophage subset provides a splenic replication niche for intracellular Salmonella. Immunity 54, 2712–2723.e6 (2021).
Mitchell, G. & Isberg, R. R. Innate immunity to intracellular pathogens: balancing microbial elimination and inflammation. Cell Host Microbe 22, 166–175 (2017).
Pilla, D. M. et al. Guanylate binding proteins promote caspase-11-dependent pyroptosis in response to cytoplasmic LPS. Proc. Natl Acad. Sci. USA 111, 6046–6051 (2014).
Clough, B. et al. p97/VCP targets Toxoplasma gondii vacuoles for parasite restriction in interferon-stimulated human cells. mSphere 8, e0051123 (2023).
Hauler, F., Mallery, D. L., McEwan, W. A., Bidgood, S. R. & James, L. C. AAA ATPase p97/VCP is essential for TRIM21-mediated virus neutralization. Proc. Natl Acad. Sci. USA 109, 19733–19738 (2012).
Matsuura, M. Structural modifications of bacterial lipopolysaccharide that facilitate Gram-negative bacteria evasion of host innate immunity. Front. Immunol. 4, 109 (2013).
Li, P. et al. Ubiquitination and degradation of GBPs by a Shigella effector to suppress host defence. Nature 551, 378–383 (2017).
Cao, S. et al. Subversion of GBP-mediated host defense by E3 ligases acquired during Yersinia pestis evolution. Nat. Commun. 13, 4526 (2022).
Wandel, M. P. et al. GBPs inhibit motility of Shigella flexneri but are targeted for degradation by the bacterial ubiquitin ligase IpaH9.8. Cell Host Microbe 22, 507–518.e5 (2017).
Tang, W. K. & Xia, D. Mutations in the human AAA(+) chaperone p97 and related diseases. Front. Mol. Biosci. 3, 79 (2016).
Valle, C. W. et al. Critical role of VCP/p97 in the pathogenesis and progression of non-small cell lung carcinoma. PLoS ONE 6, e29073 (2011).
Wrobel, L., Hoffmann, J. L., Li, X. & Rubinsztein, D. C. p37 regulates VCP/p97 shuttling and functions in the nucleus and cytosol. Sci. Adv. 10, eadl6082 (2024).
Patel, A. J. et al. Characterising the impact of pneumonia on outcome in non-small cell lung cancer: identifying preventative strategies. J. Thorac. Dis. 12, 2236–2246 (2020).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Acknowledgements
We acknowledge the Bio-safety Level 2 Facility, Confocal Microscopy Facility and FACS Facility (SR/FST/LSI-572/2013) at IIT Bombay; the Electron microscopy facility at CSIR-CCMB, Hyderabad and IIT Bombay and the Animal facility at IISc, Bengaluru, India. S.G. (17/06/2018(i) EU-V), D.H. (SPM-07/079(0293)/2019-EMR-I) and S. Roy (191620185713), and S. Rakshit (221610197857) acknowledge fellowships from CSIR and UGC, Government of India, respectively. S.S. acknowledges fellowship from PMRF, Government of India (ID: 1302049 0). S.K acknowledges research funding from the Science and Engineering Research Board, Government of India (Grant Number IPA/2020/000413). A.B. acknowledges research funding from Science and Engineering Research Board, Government of India (Grant Number SPR/2019/000808) and Ignite Life Science Foundation (Grant Number IGNITE/FG-OC/2021/004). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
A.B. conceptualized the project. S.G., S. Roy, N.B., U.K.D., P.S., D.H., S.M., M.S. and H.A. designed the methodology. S.G., S. Roy, N.B., U.K.D., P.S., D.H., S.M. and M.S. performed validation. S.G., S. Roy, P.S., D.H., S. Rakshit, S.M., M.S., J.M., R.M. and A.B. conducted formal analysis. S.G., S. Roy, N.B., U.K.D., P.S., D.H., S.D., S. Rakshit, S.M., M.S., S.S., R.S.R. and H.A. conducted investigations. J.M., D.C., R.M. and A.B. procured resources. S.G., S. Roy, P.S., J.M., R.M. and A.B. curated data. S.G. and A.B. prepared the original draft. S.G., N.B., S. Roy, S.S. and A.B. reviewed and edited the paper. S.G., S. Roy and N.B. performed visualization. S.K., J.M., D.C., R.M. and A.B. supervised the project. A.B. administered the project and acquired funding.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Microbiology thanks Melanie Hamon and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Recruitment of p97 to the bacteria is dependent on endosome damage and presence of ubiquitinable surface proteins.
A. Structural Illumination Microscopy (SIM) showing intracellular SPN (cyan) in host cells with p97 (magenta). Arrow depicts event shown in inset besides respective images. Scale bar, 5 μm. A549 cells were infected with SPN for 9 h. Infected cells were stained with DAPI to visualize pneumococcal DNA and p97 was stained with primary Ab, followed by Alexa Fluor 488 conjugated to secondary Ab. B. Percentage of p97 association with WT SPN and BgaA and PspA knockout SPN (∆pspA ∆bgaA) strains in both A549 and THP1 cells. n > 100 events, N = 3. Pneumococcus was stained with anti-enolase antibody and p97 with specific primary Ab, followed by Alexa Fluor 488 and Alexa 555 conjugated to secondary Ab, respectively. C. Graph representing fold change in the intracellular burden of WT SPN and ∆pspA ∆bgaA double mutant in WT A549 epithelial cells and cells overexpressing p97. Cells were infected with SPN (MOI ~ 25) for 9 h, and intracellular bacterial numbers were deciphered. Fold change in intracellular bacterial burden is calculated as ratio of intracellular bacterial CFU of the test (p97Overexp cells) to that of control (p97WT cells). N = 4. D. Graph representing fold change in the intracellular burden of WT SPN and ∆pspA ∆bgaA mutant in THP1 macrophages treated with siRNA specific to p97 and scramblase control. Cells were infected with SPN (MOI ~ 50) for 4 h and intracellular bacterial numbers were deciphered. Fold change in intracellular bacterial burden is calculated as ratio of intracellular bacterial CFU of the test (sip97 treated cells) to that of control (scramblase treated cells). N = 4. E. Graph representing fold change in intracellular burden of SPN ∆ply mutant in p97WT or p97Overexp host cells. Cells are infected with SPN (MOI ~ 25) for 9 h and intracellular bacterial numbers were deciphered. Fold change in intracellular bacterial burden is calculated as ratio of intracellular bacterial CFU of the test (p97Overexp cells) to that of control (p97WT cells). N = 5. F. Percentage of viability of A549 cells upon treatment with different concentrations of endomembrane perforating agent L-leucyl-L-Leucine methyl ester. N = 3. G. Percentage of FM4-64 (membrane staining dye) negative intracellular ∆ply (Pneumolysin knock-out) mutant SPN strain with or without treatment of host cells with LLOMe (3 mM). n > 100 events, N = 3. H. Percentage of p97’s association with SPN lacking pneumolysin (∆ply) and ∆ply mutant infected cells treated with LLOMe (3 mM) compared to WT SPN. n > 100 events, N = 3. I. Confocal microscopy of host A549 cells stained with p97 (green) following infection with SPN variants (red) (WT, ∆pspA ∆bgaA, ∆ply) with or without treatment with LLOMe. White arrows point towards events shown in merged insets. Scale bar, 5 µm. N = 3. Data for B-I are means ± SD of respective independent biological replicate (N). Statistical significance was assessed by two-tailed unpaired student’s t-test (nonparametric) for B, C, D, E, and G and one-way ANOVA followed by Dunnett’s test in F and H. *P < 0.05; **P < 0.01; ***P < 0.001.
Extended Data Fig. 2 siRNA mediated knockdown of gene expression and bacterial invasion in p97 knockdown condition.
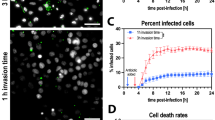
A-B. Immunoblot demonstrating the knockdown of p97 in A549, Hela and THP-1 cells (A) and PSMC6 in A549 and Hela cells (B). C-F. Immunoblot showing knockdown of NPLOC4 (C), UFD1 (D), FBXW7 (E) and FAF1 (F) in A549 cells. G-H. Immunoblot showing knockdown of RNF213 (G) and ARIH1 (H) in Hela cells. I-K. Percentage of invasion of SPN (I), STm (J) and GAS (K) in p97 downregulated cells compared to scrambled treated A549 cells. Mid-exponentially grown SPN, STm and GAS (OD600nm ~ 0.4) were used to infect monolayers of A549s (with SPN at MOI ~ 25 and GAS at MOI ~ 2) or HeLa (with STm at MOI ~ 50) for 1 h. Following elimination of extracellular bacteria with antibiotics, bacterial invasion efficiency was calculated as: (recovered CFU/initial inoculum CFU) × 100%. Data are means ± SD of N = 4, independent biological replicates. Statistical significance was assessed by two-tailed unpaired student’s t-test (nonparametric) was used for I-K, with correction for multiple comparisons. ns, non-significant.
Extended Data Fig. 3 Overexpression of p97WT or catalytically inactivated variant, p97E578Q, in host cells have unaltered effects on bacterial infections.
A. Immunoblot demonstrating the accumulation of K48-Ub substrates in the catalytically inactive p97E578Q cell line. Host cells were stably transfected with p97E578Q variant and presence of ubiquitinated proteins in cell lysates were detected by immunoblotting with anti-K48-Ub Ab. Densitometric analysis of K48-Ub band normalized to β-actin levels revealed > 2-fold enrichment of K48-Ub in p97E578Q cells compared to p97WT. B. Immunoblot showing the expression of p97-GFP in host cells. Immunoblotting was performed on cell lysates with anti-GFP and anti-p97 Abs to detect expression of p97 as well as p97-GFP fusion proteins. β-actin was used as loading control. C-E. Percentage of invasion of SPN (C), STm (D) and GAS (E) in host cells overexpressing p97 (p97Overexp) or catalytically inactive form, p97E578Q. Bacterial invasion efficiency was calculated as: (recovered CFU/initial inoculum CFU) × 100%. Data are means ± SD of N = 5, independent biological replicates. Statistical significance was assessed by one-way ANOVA followed by Dunnett’s test in C-E, with correction for multiple comparisons. ns, non-significant; **P < 0.01.
Extended Data Fig. 4 Cytosolic pathogens are targeted by p97.
A-C. Confocal micrographs showing p97 (Green) positive SPN (A), STm (B), and GAS (C) (Blue) that are devoid of membrane marker FM4-64 (Red). D-F. Confocal images depicting FM4-64 (Red) positive SPN (D), STm (E) and GAS (F) that fails to recruit p97 (Green). Arrows depict events shown in insets besides respective images. Scale bar, 5 μm. G-H. Percentage population of p97-positive bacteria that are marked with or without membrane marker FM4-64 (G) or damaged endosome marker Gal8 (H). n > 50 events. Data are means ± SD of N = 3, independent biological replicates. A549 and Hela cells were infected with SPN (MOI ~ 25), GAS (MOI ~ 2) for 9 h and STm (MOI ~ 50) for 6 h, respectively. Bacteria were stained with DAPI, membranes were marked with FM4-64, while p97 and Gal8 were detected with respective primary Ab, followed by Alexa Fluor 488 or Alexa Fluor 555 conjugated secondary Ab. Statistical significance was assessed by one-way ANOVA followed by Dunnett’s test in G, H, with correction for multiple comparisons. ***P < 0.001, ns, non-significant.
Extended Data Fig. 5 K48-polyubiquitination of bacteria is key for recruitment of p97.
A. Percentage of p97-positive bacteria in host cells treated with E1-Ub ligase inhibitor PYR41 (10 µM) compared to DMSO control. Cells were treated with PYR41 for 1 h before being infected with different pathogens. At 6-9 h, post-infection, association of intracellular bacteria (stained with DAPI) with p97 (stained with anti-p97 Ab) was assessed by quantifying confocal images. n > 100 events, N = 3. B-D. Confocal micrographs showing the association of p97 (Green) positive SPN (B), STm (C), and GAS (D) (Blue) with K48-Ub (Red) but not with K63-Ub (Magenta). E-G. Confocal images depicting K63-Ub (Magenta) positive but K48-Ub (Red) negative SPN (E), STm (F) and GAS (G) that fail to recruit p97 (Green). Arrows depict events shown in insets besides respective images. For B-G, Bacteria were stained with DAPI, p97 was conjugated to GFP, K48-Ub and K63-Ub were stained with respective primary Ab, followed by Alexa Fluor 555 or Alexa Fluor 633 conjugated secondary Ab. Scale bar, 5 μm. H. Confocal microscopy showcasing diminished K48-Ub levels in OTUB1-HA overexpressing A549 cells compared to WT A549s. K48-Ub (red) and OTUB1-HA (magenta) were detected by staining anti-K48-Ub and anti-HA Ab. Scale Bar, 5 μm. I. Percentage of association of p97 with SPN in FBXW7 downregulated host cells. FBXW7 expression in A549s were knocked down by siFBXW7, followed by infection with SPN. n > 100 events, N = 3. J. Percentage association of STm with p97 in siARIH1 and siRNF213 treated HeLa cells. While AIRH1 is reported to ubiquitinate cytosolic STm with K48-Ub chain types, RNF213 ubiquitinates LPS present on STm outer membrane. n > 100 events, N = 3. Data for A, I, J are means ± SD of respective independent biological replicate (N). Statistical significance was assessed by one-way ANOVA followed by Dunnett’s test in J or two-tailed unpaired student’s t-test (nonparametric) for A, I, with correction for multiple comparisons. ns, non-significant; *P < 0.05; **P < 0.01; ***P < 0.001.
Extended Data Fig. 6 NPLOC4 and UFD1 are the primary cofactors involved in p97’s targeting of bacteria.
A. Percentage of p97 positive intracellular SPN associating with various cofactors, such as, NPLOC4, UFD1, FAF1 or NPLOC4 positive SPN associating with UFD1. n > 50 events. Data are means ± SD of N = 3, independent biological replicates. B. Structural illumination microscopy (SIM) showing the co-localization of SPN (Cyan) with UFD1 (White), NPLOC4 (Magenta) and p97 (Yellow). Scale bar, 1 μm. 3D reconstruction of the merged image using IMARIS is also represented. Bacteria were stained with DAPI, p97 was conjugated to GFP, NPLOC4 and UFD1 were stained with respective primary Ab, followed by Alexa Fluor 633 or 555 conjugated to secondary Ab. C. Immunoblot showcasing the accumulation of K48-Ub substrates in host cells expressing p97∆54-241. Host cells were stably transfected with truncated p97 variant and inability to clear ubiquitinated proteins was detected by immunoblotting with anti-K48-Ub Ab. Densitometric analysis of K48-Ub band normalized to β-actin levels revealed ~ 1.4-fold enrichment of K48-Ub in p97∆54-241 cells compared to p97WT. D. Quantitative analysis showcasing association of p97 with intracellular SPN in host cells expressing truncated p97∆54-241 compared to wild-type. n > 100 bacteria/coverslip. Data are means ± SD of N = 3, independent biological replicates. E. Fold change in intracellular burden of SPN in host cells expressing truncated p97∆54-241 compared to wild-type cells. Host cells are infected with SPN (MOI ~ 25) for 9 h and fold change in intracellular bacterial burden is depicted as ratio of intracellular bacterial CFU of the test to that of control at specified time point. Data are means ± SD of N = 6, independent biological replicates. Statistical significance was assessed by Two-tailed unpaired student’s t-test (nonparametric) for D, E, with correction for multiple comparisons. **P < 0.01; ***P < 0.001.
Extended Data Fig. 7 Coordination of p97 with proteasome is required for pathogen elimination.
A-C. Confocal micrographs showing the association of p97 (Green) positive SPN (A), STm (B), and GAS (C) (Blue) with proteasomal marker, β7 (Red). Arrows depict events shown in insets besides respective images. Scale bar, 5 μm. D. Percentage association of p97-positive pathogens with the proteasome (marked by β7). n > 100 events examined for three independent experiments. E. Percentage of β7 (Proteasomal marker) positive intracellular bacteria in host cells treated with sip97 compared with siControl (Scramblase). A549 and Hela cells were infected with SPN, GAS for 9 h and STm for 6 h, respectively. Bacteria were stained with DAPI, p97 and β7 with respective primary Ab, followed by Alexa Fluor 488 or Alexa Fluor 555 conjugated to secondary Ab. n > 100 events, data are means ± SD of N = 3 independent biological replicates. F. Graph depicting the percentage of p97-positive bacteria upon treatment with proteasomal inhibitor MG132, compared to DMSO control. Bacteria and p97 were stained with specific primary antibodies (SPN with anti-enolase, STm with anti-LPS and GAS with anti-GAS capsule), followed by Alexa 488 and Alexa 555 conjugated secondary antibody staining. n > 100 events, data are means ± SD of N = 3 independent biological replicates. G. Fold change in intracellular burden of SPN, STm and GAS upon treatment of host cells with either Scramblase (siControl) or sip97 or siControl + MG132 and sip97 + MG132. Fold change in intracellular bacterial burden is depicted as ratio of intracellular bacterial CFU of the test to that of control at specified time points. Data are means ± SD of N = 4 independent biological replicates. Statistical significance was assessed by Two-tailed unpaired student’s t-test (nonparametric) in E and F and one-way ANOVA followed by Dunnett’s test in G, with correction for multiple comparisons. *P < 0.05; **P < 0.01, ***P < 0.001.
Extended Data Fig. 8 Transmission electron microscopy of SPN showcasing membranolytic property of p97.
A, D, F. Transmission electron micrographic images showing membrane disruptions in SPN cells following in vitro ubiquitination and treatment with p97. Scale bar as depicted. Zoomed in images of various areas in (A), (D) and (F) are shown in (B-C), (E) and (G), respectively. H. Immunogold-TEM image showing deposition of p97 on SPN surface following infection in A549 cells. Scale bar, 500 nm. I, J. Zoomed in view of the areas in H marked as (1) and (2) are shown in I and J, respectively, showcasing presence of p97 specific gold particles (25 nm) (blue arrows) and pinching of the peptidoglycan layer (red arrows) from the same area on SPN surface.
Extended Data Fig. 9 p97 unfolds and translocates along BgaA-T.
A-B. SDS PAGE of p97-Strep TagII (A) and BgaA-T (B) purified using Ni-NTA column from cell lysate of E. coli(DE3) cells expressing the proteins. Molecular weight of markers is mentioned in the side of the SDS gels. CFE: Cell-free extract; EF: Elution fraction. Protein expression was induced using IPTG (0.2 mM). C. Immunoblot showcasing the formation and binding of p97-NPLOC4-UFD1 complex on Strep-beads. D. Immunostaining of BgaA-T (a truncated version of BgaA, consisting of amino acids 1 to 1049) deposited on a hydrophilic coverslip using anti-(His)6 antibody. Scale bar, 20 µm. E. Beads (unconjugated or conjugated with p97) were trapped and brought close to the coverslip surface coated with BgaA-T or ubiqutinated BgaA-T. The percentage of beads that bound the surface was calculated and plotted for each of the conditions described in the figure. n > 20 beads. F. Distance from trap centre versus time plot the trap centre giving multiple force generating events (one of which is depicted in the green box). Representative beads conjugated with p97WT (Left) and p97E578Q (Right) are shown. Orange arrows indicate the time points at which piezo was moved. On moving the piezo, the p97E578Q mutant binds the surface but keeps falling back to the trap, however only a few beads escaped the trap. Once the piezo is moved, the p97WT bead binds the surface and extracts the protein. Hence the majority of the beads escape the trap (as shown). G. Streptavidin bead was moved in 20 nm steps in + and - Z positions and images were acquired at each step. A calibration curve – position versus intensity of greyscale histogram, was generated using the obtained values to calculate displacement for protein-conjugated beads, when they bind to BgaA surface. Blue dots represent intensity values obtained with only bead. Red triangles and green triangles are values obtained with p97WT and p97E578Q beads, respectively. H. Time-lapse images of representative p97WT and p97E578Q tagged bead translocating towards BgaA-T laden coverslip without moving the piezo stage. I. Graph representing the translocation kinetics of p97WT and p97E578Q beads towards the BgaA-T coated coverslip.
Extended Data Fig. 10 VCP/p97 targets bacterial surface proteins to curb sepsis in mice.
A-B. Graph demonstrating the comparison of bacterial burden in blood (A) and spleen (B) of mice infected with SPN lacking BgaA and PspA for 12 h. Animals were pretreated with either NMS-873 (0.02 mg/kg) or vehicle before infection. C-D. Graph depicting the cytokine levels in blood (C) and spleen (D) of mice at 12 h.p.i. with ΔpspAΔbgaA mutant. 1 h prior to infection, animals were treated with either DMSO (vehicle) or NMS-873 (0.02 mg/kg). For all the experiments, n > 4 mice per group, experiments were repeated twice, and data is collectively represented and means of ± SD of 8 mice per group. Statistical significance was assessed by two-way ANOVA followed by Bonferroni test for A-D, with correction for multiple comparisons. ns, non-significant.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8, Tables 1–3, Movies 1–4 and unprocessed immunoblots depicted in the supplementary figures.
Supplementary Video 1
Time-lapse confocal microscopy of SPN killing by p97. Time-lapse confocal microscopy demonstrating gradual degradation of tagRFP-SPN (red) upon association with GFP-p97 (green) in A549 host cells (white arrowhead). tagRFP-SPN (red) devoid of p97 (blue arrowhead), present in the same field shown as a control. Scale bar, 0.5 μM. A montage of the representative stills corresponding to the movie are presented in Supplementary Fig. 1a.
Supplementary Video 2
Time-lapse confocal microscopy of intracellular SPN:∆pspA∆bgaA. Time-lapse confocal microscopy demonstrating intracellular persistence of SPN:∆pspA∆bgaA mutant strain (red; stained with SYTO Deep Red Nucleic acid stain for Live cells; Invitrogen, S34900), which was devoid of GFP-p97 (green) in A549 host cells (white arrowhead). Scale bar, 0.5 μM. A montage of the representative stills corresponding to the movie are presented in Supplementary Fig.1e.
Supplementary Video 3
Time-lapse confocal microscopy of intracellular SPN:∆ply. Time-lapse confocal microscopy demonstrating persistence of SPN:∆ply (red), stained with SYTO Deep Red nucleic acid stain for live cells (Invitrogen, S34900), which was devoid of GFP-p97 (green) in A549 host cells (white arrowhead). Scale bar, 0.5 μM. A montage of the representative stills corresponding to the movie are presented in Supplementary Fig.1e.
Supplementary Video 4
Molecular dynamic simulation of BgaA pulling by p97. Steered molecular dynamic (SMD) simulation of p97-mediated pulling of BgaA, covalently attached to peptidoglycan surface. Severe deformation of peptidoglycan layer was observed after complete unfolding of BgaA. The snapshot is represented in Fig. 5a.
Source data
Source Data Fig. 1
Unprocessed western blots.
Source Data Fig. 1
Numerical data for graphs.
Source Data Fig. 2
Numerical data for graphs.
Source Data Fig. 3
Numerical data for graphs.
Source Data Fig. 4
Unprocessed western blots.
Source Data Fig. 4
Numerical data for graphs.
Source Data Fig. 5
Numerical data for graphs.
Source Data Fig. 6
Numerical data for graphs.
Source Data Extended Data Fig. 1
Numerical data for graphs.
Source Data Extended Data Fig. 2
Unprocessed western blots.
Source Data Extended Data Fig. 2
Numerical data for graphs.
Source Data Extended Data Fig. 3
Unprocessed western blots.
Source Data Extended Data Fig. 3
Numerical data for graphs.
Source Data Extended Data Fig. 4
Numerical data for graphs.
Source Data Extended Data Fig. 5
Numerical data for graphs.
Source Data Extended Data Fig. 6
Unprocessed western blots.
Source Data Extended Data Fig. 6
Numerical data for graphs.
Source Data Extended Data Fig. 7
Numerical data for graphs.
Source Data Extended Data Fig. 9
Unprocessed western blots.
Source Data Extended Data Fig. 9
Numerical data for graphs.
Source Data Extended Data Fig. 10
Numerical data for graphs.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ghosh, S., Roy, S., Baid, N. et al. Host AAA-ATPase VCP/p97 lyses ubiquitinated intracellular bacteria as an innate antimicrobial defence. Nat Microbiol 10, 1099–1114 (2025). https://doi.org/10.1038/s41564-025-01984-y
Received:
Accepted:
Published:
Version of record:
Issue date:
DOI: https://doi.org/10.1038/s41564-025-01984-y
This article is cited by
-
Extract to lyse bacterial pathogens
Nature Microbiology (2025)